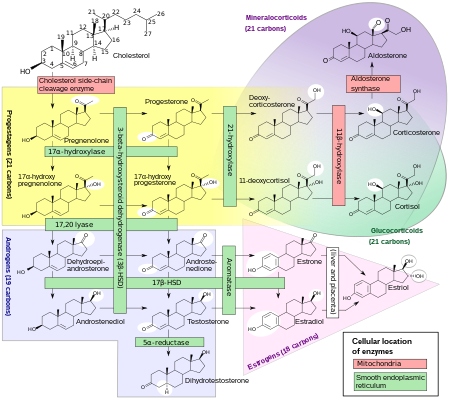
Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the major progestogen in the body. Progesterone has a variety of important functions in the body. It is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid.

Sex hormones, also known as sex steroids, gonadocorticoids and gonadal steroids, are steroid hormones that interact with vertebrate steroid hormone receptors. The sex hormones include the androgens, estrogens, and progestogens. Their effects are mediated by slow genomic mechanisms through nuclear receptors as well as by fast nongenomic mechanisms through membrane-associated receptors and signaling cascades. The polypeptide hormones luteinizing hormone, follicle-stimulating hormone and gonadotropin-releasing hormone – each associated with the gonadotropin axis – are usually not regarded as sex hormones, although they play major sex-related roles.
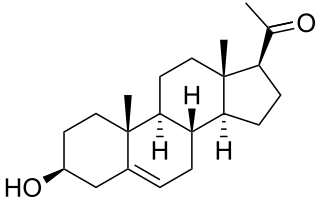
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids. In addition, pregnenolone is biologically active in its own right, acting as a neurosteroid.
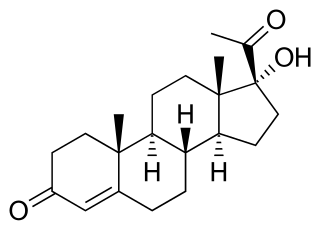
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.

5α-Dihydroprogesterone is an endogenous progestogen and neurosteroid that is synthesized from progesterone. It is also an intermediate in the synthesis of allopregnanolone and isopregnanolone from progesterone.

Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. It is taken by mouth.

Epipregnanolone, also known as 3β-hydroxy-5β-pregnan-20-one, 3β,5β-tetrahydroprogesterone, or 3β,5β-THP, is an endogenous neurosteroid. It acts as a negative allosteric modulator of the GABAA receptor and reverses the effects of potentiators like allopregnanolone. Epipregnanolone is biosynthesized from progesterone by the actions of 5β-reductase and 3β-hydroxysteroid dehydrogenase, with 5β-dihydroprogesterone as the intermediate in this two-step transformation.

20α-Dihydroprogesterone (20α-DHP), also known as 20α-hydroxyprogesterone (20α-OHP), is a naturally occurring, endogenous progestogen. It is a metabolite of progesterone, formed by the 20α-hydroxysteroid dehydrogenases (20α-HSDs) AKR1C1, AKR1C2, and AKR1C3 and the 17β-hydroxysteroid dehydrogenase (17β-HSD) HSD17B1. 20α-DHP can be transformed back into progesterone by 20α-HSDs and by the 17β-HSD HSD17B2. HSD17B2 is expressed in the human endometrium and cervix among other tissues. In animal studies, 20α-DHP has been found to be selectively taken up into and retained in target tissues such as the uterus, brain, and skeletal muscle.

5β-Dihydroprogesterone is an endogenous neurosteroid and an intermediate in the biosynthesis of pregnanolone and epipregnanolone from progesterone. It is synthesized from progesterone by the enzyme 5β-reductase.
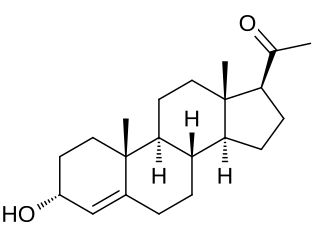
3α-Dihydroprogesterone (3α-DHP), also known as 3α-hydroxyprogesterone, as well as pregn-4-en-3α-ol-20-one, is an endogenous neurosteroid. It is biosynthesized by 3α-hydroxysteroid dehydrogenase from progesterone. 3α-DHP has been found to act as a positive allosteric modulator of the GABAA receptor and is described as being as active as allopregnanolone in regard to this action. In accordance, it has anxiolytic effects in animals. 3α-DHP has also been found to inhibit the secretion of follicle-stimulating hormone (FSH) from the rat pituitary gland, demonstrating possible antigonadotropic properties. Unlike the case of most other inhibitory neurosteroids, 3α-DHP production is not blocked by 5α-reductase inhibitors like finasteride. No data were available on the progestogenic activity of 3α-DHP as of 1977. Levels of 5α-DHP have been quantified.

3β-Dihydroprogesterone (3β-DHP), also known as 3β-hydroxyprogesterone, or pregn-4-en-3β-ol-20-one, is an endogenous steroid. It is biosynthesized by 3β-hydroxysteroid dehydrogenase from progesterone. Unlike 3α-dihydroprogesterone (3α-DHP), 3β-DHP does not act as a positive allosteric modulator of the GABAA receptor, which is in accordance with the fact that other 3β-hydroxylated progesterone metabolites such as isopregnanolone and epipregnanolone similarly do not act as potentiators of this receptor and instead inhibit it as well as reverse the effects of potentiators like allopregnanolone. 3β-DHP has been reported to possess about the same potency as progesterone in a bioassay of progestogenic activity, whereas 3α-DHP was not assessed.

Retroprogesterone, also known as 9β,10α-progesterone or as 9β,10α-pregn-4-ene-3,20-dione, is a progestin which was never marketed. It is a stereoisomer of the naturally occurring progestogen progesterone, in which the hydrogen atom at the 9th carbon is in the α-position instead of the β-position and the methyl group at the 10th carbon is in the β-position instead of the α-position. In other words, the atom positions at the two carbons have been reversed relative to progesterone, hence the name retroprogesterone. This reversal results in a "bent" configuration in which the plane of rings A and B is orientated at a 60° angle below the rings C and D. This configuration is ideal for interaction with the progesterone receptor, with retroprogesterone binding with high affinity to this receptor. However, the configuration is not as ideal for binding to other steroid hormone receptors, and as a result, retroprogesterone derivatives have increased selectivity for the progesterone receptor relative to progesterone.

16α-Hydroxyprogesterone (16α-OHP), also known as 16α-hydroxypregn-4-ene-3,20-dione, is a minor endogenous progestogen steroid hormone and a metabolite of progesterone that is formed in lower amounts than 17α-hydroxyprogesterone (17α-OHP). It occurs in micromolar concentrations and its physiological relevance hence is questionable. However, it may accumulate in target tissues and could have a physiological role in the reproductive system and mammary gland development as well as the cardiovascular and central nervous systems.

5α-Dihydronorethisterone is a major active metabolite of norethisterone (norethindrone). Norethisterone is a progestin with additional weak androgenic and estrogenic activity. 5α-DHNET is formed from norethisterone by 5α-reductase in the liver and other tissues.
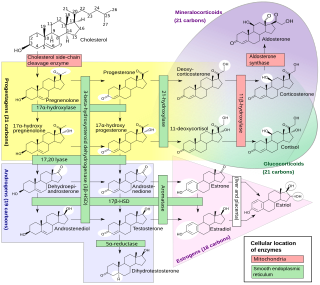
Steroidogenic enzymes are enzymes that are involved in steroidogenesis and steroid biosynthesis. They are responsible for the biosynthesis of the steroid hormones, including sex steroids and corticosteroids, as well as neurosteroids, from cholesterol. Steroidogenic enzymes are most highly expressed in classical steroidogenic tissues, such as the testis, ovary, and adrenal cortex, but are also present in other tissues in the body.

20β-Dihydroprogesterone (20β-DHP), also known as 20β-hydroxyprogesterone (20β-OHP), is an endogenous metabolite of progesterone which is formed by 20β-hydroxysteroid dehydrogenase (20β-HSD). It is a progestogen similarly to progesterone, with about 20 to 50% of the progestogenic activity of progesterone. It can be converted by 20β-HSD into progesterone in the uterus. The effects of 20β-HSD on the uterus, mammary glands, and in maintaining pregnancy have been studied. The progestogenic activity of 20β-HSD has also been characterized in women.
The pharmacology of progesterone, a progestogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.