The adrenal glands are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis.

Renin, also known as an angiotensinogenase, is an aspartic protease protein and enzyme secreted by the kidneys that participates in the body's renin-angiotensin-aldosterone system (RAAS)—also known as the renin-angiotensin-aldosterone axis—that increases the volume of extracellular fluid and causes arterial vasoconstriction. Thus, it increases the body's mean arterial blood pressure.

The renin-angiotensin system (RAS), or renin-angiotensin-aldosterone system (RAAS), is a hormone system that regulates blood pressure, fluid, and electrolyte balance, and systemic vascular resistance.

Angiotensin is a peptide hormone that causes vasoconstriction and an increase in blood pressure. It is part of the renin–angiotensin system, which regulates blood pressure. Angiotensin also stimulates the release of aldosterone from the adrenal cortex to promote sodium retention by the kidneys.

The adrenal cortex is the outer region and also the largest part of the adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones. It is also a secondary site of androgen synthesis.
Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume. When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.

Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.

Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances. The primary mineralocorticoid is aldosterone.
Adrenal insufficiency is a condition in which the adrenal glands do not produce adequate amounts of steroid hormones. The adrenal glands—also referred to as the adrenal cortex—normally secrete glucocorticoids, mineralocorticoids, and androgens. These hormones are important in regulating blood pressure, electrolytes, and metabolism as a whole. Deficiency of these hormones leads to symptoms ranging from abdominal pain, vomiting, muscle weakness and fatigue, low blood pressure, depression, mood and personality changes to organ failure and shock. Adrenal crisis may occur if a person having adrenal insufficiency experiences stresses, such as an accident, injury, surgery, or severe infection; this is a life-threatening medical condition resulting from severe deficiency of cortisol in the body. Death may quickly follow.
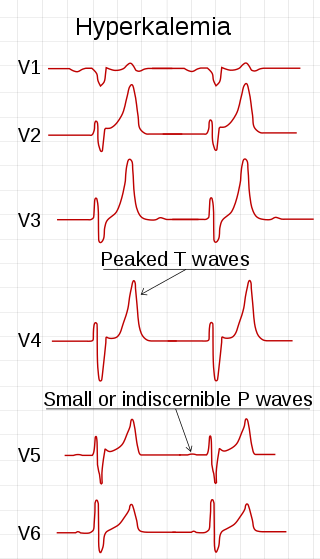
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5 mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasionally when severe it can cause palpitations, muscle pain, muscle weakness, or numbness. Hyperkalemia can cause an abnormal heart rhythm which can result in cardiac arrest and death.

Potassium-sparing diuretics or antikaliuretics refer to drugs that cause diuresis without causing potassium loss in the urine. They are typically used as an adjunct in management of hypertension, cirrhosis, and congestive heart failure. The steroidal aldosterone antagonists can also be used for treatment of primary hyperaldosteronism. Spironolactone, a steroidal aldosterone antagonist, is also used in management of female hirsutism and acne from PCOS or other causes.

Hypoaldosteronism is an endocrinological disorder characterized by decreased levels of the hormone aldosterone. Similarly, isolated hypoaldosteronism is the condition of having lowered aldosterone without corresponding changes in cortisol.

The zona glomerulosa of the adrenal gland is the most superficial layer of the adrenal cortex, lying directly beneath the renal capsule. Its cells are ovoid and arranged in clusters or arches.

Hyperaldosteronism is a medical condition wherein too much aldosterone is produced. High aldosterone levels can lead to lowered levels of potassium in the blood (hypokalemia) and increased hydrogen ion excretion (alkalosis). Aldosterone is normally produced in the adrenal glands.
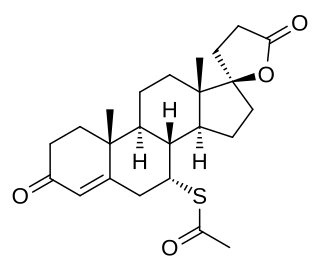
A mineralocorticoid receptor antagonist or aldosterone antagonist, is a diuretic drug which antagonizes the action of aldosterone at mineralocorticoid receptors. This group of drugs is often used as adjunctive therapy, in combination with other drugs, for the management of chronic heart failure. Spironolactone, the first member of the class, is also used in the management of hyperaldosteronism and female hirsutism. Most antimineralocorticoids, including spironolactone, are steroidal spirolactones. Finerenone is a nonsteroidal antimineralocorticoid.

Bartter syndrome (BS) is a rare inherited disease characterised by a defect in the thick ascending limb of the loop of Henle, which results in low potassium levels (hypokalemia), increased blood pH (alkalosis), and normal to low blood pressure. There are two types of Bartter syndrome: neonatal and classic. A closely associated disorder, Gitelman syndrome, is milder than both subtypes of Bartter syndrome.
Pseudohyperaldosteronism is a medical condition which mimics the effects of elevated aldosterone (hyperaldosteronism) by presenting with high blood pressure, low blood potassium levels (hypokalemia), metabolic alkalosis, and low levels of plasma renin activity (PRA). However, unlike hyperaldosteronism, this conditions exhibits low or normal levels of aldosterone in the blood. Causes include genetic disorders, acquired conditions, metabolic disorders, and dietary imbalances including excessive consumption of licorice. Confirmatory diagnosis depends on the specific cause and may involve blood tests, urine tests, or genetic testing; however, all forms of this condition exhibit abnormally low concentrations of both plasma renin activity (PRA) and plasma aldosterone concentration (PAC) which differentiates this group of conditions from other forms of secondary hypertension. Treatment is tailored to the specific cause and focuses on symptom control, blood pressure management, and avoidance of triggers.

Aldosterone synthase, also called steroid 18-hydroxylase, corticosterone 18-monooxygenase or P450C18, is a steroid hydroxylase cytochrome P450 enzyme involved in the biosynthesis of the mineralocorticoid aldosterone and other steroids. The enzyme catalyzes sequential hydroxylations of the steroid angular methyl group at C18 after initial 11β-hydroxylation. It is encoded by the CYP11B2 gene in humans.
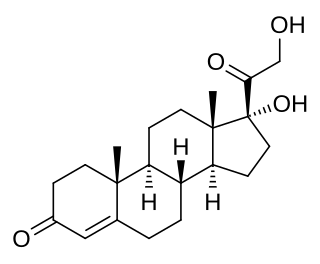
11-Deoxycortisol, also known as cortodoxone (INN), cortexolone as well as 17α,21-dihydroxyprogesterone or 17α,21-dihydroxypregn-4-ene-3,20-dione, is an endogenous glucocorticoid steroid hormone, and a metabolic intermediate toward cortisol. It was first described by Tadeusz Reichstein in 1938 as Substance S, thus has also been referred to as Reichstein's Substance S or Compound S.
Glucocorticoid remediable aldosteronism also describable as aldosterone synthase hyperactivity, is an autosomal dominant disorder in which the increase in aldosterone secretion produced by ACTH is no longer transient.