| |
| Clinical data | |
|---|---|
| Trade names | Ovulen, Demulen, others |
| Other names | Ethynodiol diacetate; Norethindrol diacetate; 3β-Hydroxynorethisterone 3β,17β-diacetate; [1] 17α-Ethynylestr-4-ene-3β,17β-diyl diacetate; CB-8080; SC-11800 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin; Progestogen ester |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.496 |
| Chemical and physical data | |
| Formula | C24H32O4 |
| Molar mass | 384.516 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Etynodiol diacetate, or ethynodiol diacetate, sold under the brand name Ovulen among others, is a progestin medication which is used in birth control pills. [4] [5] [6] The medication is available only in combination with an estrogen. [7] It is taken by mouth. [8]
Contents
- Medical uses
- Side effects
- Pharmacology
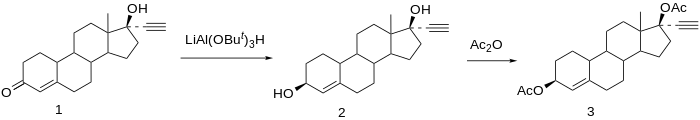
- Chemistry
- Synthesis
- History
- Society and culture
- Generic names
- Brand names
- Availability
- References
Etynodiol diacetate is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone. [9] [10] It has weak androgenic and estrogenic activity and no other important hormonal activity. [11] [12] [13] The medication is a prodrug of norethisterone in the body, with etynodiol occurring as an intermediate. [9] [10] [14]
Etynodiol, a related compound, was discovered in 1954, and etynodiol diacetate was introduced for medical use in 1965. [15] [16] The combination ethynodiol with mestranol (Ovulen) was approved for medical use in the United States in 1966. [17] The combination ethinylestradiol with ethynodiol (Demulen) was approved for medical use in the United States in 1970. [18]
In 2021, the combination with ethinylestradiol was the 276th most commonly prescribed medication in the United States, with more than 800,000 prescriptions. [19] [20]