 | |
| Names | |
|---|---|
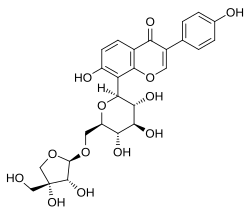
| IUPAC name 8-[β-D-Apiofuranosyl-(1→6)-β-D-glucopyranosyloxy]-4′,7-dihydroxyisoflavone | |
| Systematic IUPAC name 8-[(2S,3R,4R,5S,6R)-6-({[(2R,3R,4R)-3,4-Dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxy}methyl)-3,4,5-trihydroxyoxan-2-yl]-7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names Daidzein 8-C-(6-apiofuranosylglucoside) | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C26H28O13 | |
| Molar mass | 548.497 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Mirificin, also known as daidzein 8-C-(6-apiofuranosylglucoside), is an isoflavone that is found in Pueraria mirifica and Pueraria lobata . [1] [2] It has estrogenic activity and hence is a phytoestrogen. [1] [2]