 | |
| Clinical data | |
|---|---|
| Trade names | Fablyn |
| Routes of administration | By mouth |
| Drug class | Selective estrogen receptor modulator |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
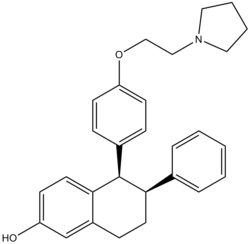
| Formula | C28H31NO2 |
| Molar mass | 413.55 g/mol 563.64 g/mol (tartrate) g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Lasofoxifene, sold under the brand name Fablyn, is a nonsteroidal selective estrogen receptor modulator (SERM) which is marketed by Pfizer in Lithuania and Portugal for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy, [1] [2] and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND). It also appears to have had a statistically significant effect of reducing breast cancer in women according to a study published in The Journal of the National Cancer Institute.