 | |
| Clinical data | |
|---|---|
| Other names | GDC-0810, ARN-810, RG-6046, RO-7056118 |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
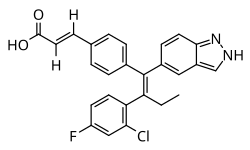
| Formula | C26H20ClFN2O2 |
| Molar mass | 446.91 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Brilanestrant (INN; developmental codes GDC-0810, ARN-810, RG-6046, and RO-7056118) is a nonsteroidal combined selective estrogen receptor modulator (SERM) and selective estrogen receptor degrader (SERD) that was discovered by Aragon Pharmaceuticals and was under development by Genentech for the treatment of locally advanced or metastatic estrogen receptor (ER)-positive breast cancer. [1] [2] [3] [4] [5]
Contents
Development of brilanestrant was discontinued by Roche in April 2017. [6] It reached phase II clinical trials for the treatment of breast cancer prior to the discontinuation of its development. [2] [5]