 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ˈkwɜːrsɪtɪn/ |
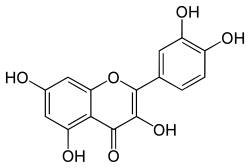
| IUPAC name 3,3′,4′,5,7-Pentahydroxyflavone | |
| Systematic IUPAC name 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one | |
| Other names 5,7,3′,4′-flavon-3-ol, Sophoretin, Meletin, Quercetine, Xanthaurine, Quercetol, Quercitin, Quertine, Flavin meletin | |
| Identifiers | |
3D model (JSmol) | |
| 317313 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.807 |
| EC Number |
|
| 579210 | |
| KEGG | |
PubChem CID | |
| UNII |
|
| UN number | 2811 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C15H10O7 | |
| Molar mass | 302.236 g/mol |
| Appearance | yellow crystalline powder [1] |
| Density | 1.799 g/cm3 |
| Melting point | 316 °C (601 °F; 589 K) |
| Practically insoluble in water; soluble in aqueous alkaline solutions [1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |

Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. [2] [3] It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.
