
Estrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal activity: estrone (E1), estradiol (E2), and estriol (E3). Estradiol, an estrane, is the most potent and prevalent. Another estrogen called estetrol (E4) is produced only during pregnancy.

Estriol (E3), also spelled oestriol, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estrone. Levels of estriol in women who are not pregnant are almost undetectable. However, during pregnancy, estriol is synthesized in very high quantities by the placenta and is the most produced estrogen in the body by far, although circulating levels of estriol are similar to those of other estrogens due to a relatively high rate of metabolism and excretion. Relative to estradiol, both estriol and estrone have far weaker activity as estrogens.

Selective estrogen receptor modulators (SERMs), also known as estrogen receptor agonists/antagonists (ERAAs), are a class of drugs that act on estrogen receptors (ERs). Compared to pure ER agonists–antagonists, SERMs are more tissue-specific, allowing them to selectively inhibit or stimulate estrogen-like action in various tissues.

Estrogen receptor beta (ERβ) also known as NR3A2 is one of two main types of estrogen receptor—a nuclear receptor which is activated by the sex hormone estrogen. In humans ERβ is encoded by the ESR2 gene.

Estrone sulfate, also known as E1S, E1SO4 and estrone 3-sulfate, is a natural, endogenous steroid and an estrogen ester and conjugate.

Estetrol (E4), or oestetrol, is one of the four natural estrogenic steroid hormones found in humans, along with estrone (E1), estradiol (E2), and estriol (E3). Estetrol is a major estrogen in the body. In contrast to estrone and estradiol, estetrol is a native estrogen of fetal life. Estetrol is produced exclusively by the fetal liver and is found in detectable levels only during pregnancy, with relatively high levels in the fetus and lower levels in the maternal circulation.

Estriol succinate, sold under the brand name Synapause among others, is an estrogen medication which is used in the treatment of menopausal symptoms. It is taken by mouth, in through the vagina, and by injection.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.

Estradiol/norethisterone (E2/NET), tentative brand name Netagen or Netagen 403, was a combination of estradiol (E2), an estrogen, and norethisterone (NET), a progestin, which was studied as a birth control pill to prevent pregnancy in women. It was taken by mouth and contained 4 mg micronized E2 and 3 mg NET per tablet. The medication was developed by Novo Pharmaceuticals in Denmark and was never marketed.
Combined birth control pills that contain natural estradiol or an estradiol ester include:

16α-Iodo-E2, or 16α-iodoestradiol, is a synthetic, steroidal, potent estrogen with slight preference for the ERα over the ERβ that is used in scientific research. The KD of 16α-iodo-E2 for the ERα is 0.6 nM and for the ERβ is 0.24 nM, a 4-fold difference in affinity, whereas estradiol is considered to have similar affinity for the two receptor subtypes. Unlike the case of the much weaker estriol (16α-hydroxyestradiol), 16α-iodo-E2 is considered to be equipotent with estradiol in terms of estrogenic activity. Radiolabeled [16α-125I]iodo-E2 has been employed in imaging to study the estrogen receptor.

2-Hydroxyestradiol (2-OHE2), also known as estra-1,3,5(10)-triene-2,3,17β-triol, is an endogenous steroid, catechol estrogen, and metabolite of estradiol, as well as a positional isomer of estriol.

16α-Hydroxyestrone (16α-OH-E1), or hydroxyestrone, also known as estra-1,3,5(10)-triene-3,16α-diol-17-one, is an endogenous steroidal estrogen and a major metabolite of estrone, as well as an intermediate in the biosynthesis of estriol. It is a potent estrogen similarly to estrone, and it has been suggested that the ratio of 16α-hydroxyestrone to 2-hydroxyestrone, the latter being much less estrogenic in comparison and even antiestrogenic in the presence of more potent estrogens like estradiol, may be involved in the pathophysiology of breast cancer. Conversely, 16α-hydroxyestrone may help to protect against osteoporosis.

Estradiol/progesterone (E2/P4), sold under the brand name Bijuva among others, is a combined estrogen and progestogen medication which is used in the treatment of menopausal symptoms in postmenopausal women. It contains estradiol, an estrogen, and progesterone, a progestogen, and is available in both oral and intramuscular formulations. E2/P4 differs from other estrogen–progestogen formulations in that the sex-hormonal agents used are bioidentical.

An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women. They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol. Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone.

Estriol (E3), sold under the brand name Ovestin among others, is an estrogen medication and naturally occurring steroid hormone which is used in menopausal hormone therapy. It is also used in veterinary medicine as Incurin to treat urinary incontinence due to estrogen deficiency in dogs. The medication is taken by mouth in the form of tablets, as a cream that is applied to the skin, as a cream or pessary that is applied in the vagina, and by injection into muscle.

Dimethylstilbestrol (DMS) is a nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol which was never marketed. It is a so-called "weak", "impeded", or "short-acting" estrogen similarly to estriol and meso-butoestrol. The affinity of DMS for the ER was reported as about 10% of that of estradiol. For comparison, diethylstilbestrol had 140% of the affinity of estradiol for the ER.
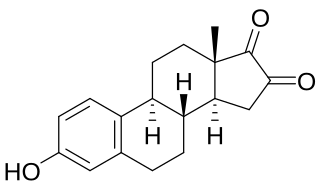
16-Ketoestrone is an endogenous estrogen related to 16α-hydroxyestrone and 16β-hydroxyestrone. In contrast to 16α-hydroxyestrone and 16β-hydroxyestrone, but similarly to 16-ketoestradiol, 16-ketoestrone is a very weak estrogen with less than 1/1000 the estrogenic potency of estrone in the uterus. 16-Ketoestrone has been reported to act as an inhibitor of 17β-hydroxysteroid dehydrogenases. 16-Ketoestrone can be converted by 16α-hydroxysteroid dehydrogenase into estriol in the body.

meso-Butestrol, also known as 2,3-bis(4-hydroxyphenyl)butane, is a synthetic nonsteroidal estrogen which was never marketed. It is a so-called "short-acting" or "impeded" estrogen. meso-Butestrol is structurally related to diethylstilbestrol and other stilbestrols. The fully potent counterpart to meso-butestrol is meso-hexestrol, analogously to the relationship of dimethylstilbestrol to diethylstilbestrol.

ent-Estradiol (ent-E2), or 1-estradiol (1-E2), is an estrogen and the 1-enantiomorph of estradiol. It is a so-called "short-acting" or "impeded" estrogen, similarly to estriol, 17α-estradiol, and dimethylstilbestrol.














