| |
 | |
| Names | |
|---|---|
| IUPAC name Cholesta-5,7-dien-3β-ol | |
| Systematic IUPAC name (1R,3aR,7S,9aR,9bS,11aR)-9a,11a-Dimethyl-1-[(2R)-6-methylheptan-2-yl]-2,3,3a,6,7,8,9,9a,9b,10,11,11a-dodecahydro-1H-cyclopenta[a]phenanthren-7-ol | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.456 |
| MeSH | 7-dehydrocholesterol |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C27H44O | |
| Molar mass | 384.638 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |

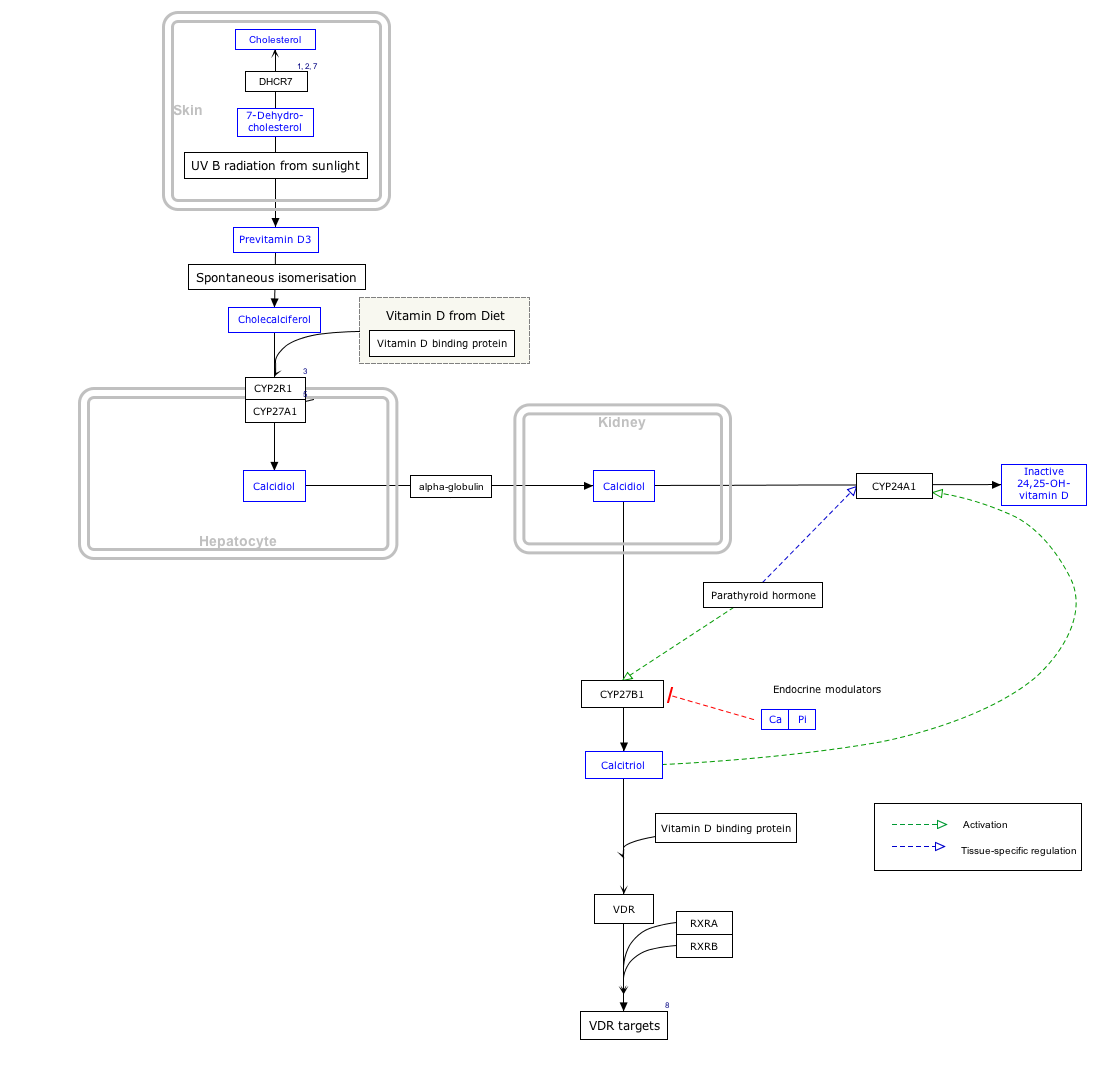
7-Dehydrocholesterol (7-DHC) is a zoosterol that functions in the serum as a cholesterol precursor, and is photochemically converted to vitamin D3 in the skin, therefore functioning as provitamin-D3. The presence of this compound in human skin enables humans to manufacture vitamin D3 (cholecalciferol). Upon exposure to ultraviolet UV-B rays in the sun light, 7-DHC is converted into vitamin D3 via previtamin D3 as an intermediate isomer. It is also found in the milk of several mammalian species. [1] [2] Lanolin, a waxy substance that is naturally secreted by wool-bearing mammals, contains 7-DHC which is converted into vitamin D by sunlight and then ingested during grooming as a nutrient. In insects 7-dehydrocholesterol is a precursor for the hormone ecdysone, required for reaching adulthood. [3] 7-DHC was discovered by Nobel-laureate organic chemist Adolf Windaus.