
Ultraviolet (UV) light is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights.

7-Dehydrocholesterol (7-DHC) is a zoosterol that functions in the serum as a cholesterol precursor, and is photochemically converted to vitamin D3 in the skin, therefore functioning as provitamin-D3. The presence of this compound in human skin enables humans to manufacture vitamin D3 (cholecalciferol). Upon exposure to ultraviolet UV-B rays in the sun light, 7-DHC is converted into vitamin D3 via previtamin D3 as an intermediate isomer. It is also found in the milk of several mammalian species. Lanolin, a waxy substance that is naturally secreted by wool-bearing mammals, contains 7-DHC which is converted into vitamin D by sunlight and then ingested during grooming as a nutrient. In insects 7-dehydrocholesterol is a precursor for the hormone ecdysone, required for reaching adulthood. 7-DHC was discovered by Nobel-laureate organic chemist Adolf Windaus.

Sunscreen, also known as sunblock or sun cream, is a photoprotective topical product for the skin that helps protect against sunburn and prevent skin cancer. Sunscreens come as lotions, sprays, gels, foams, sticks, powders and other topical products. Sunscreens are common supplements to clothing, particularly sunglasses, sunhats and special sun protective clothing, and other forms of photoprotection.
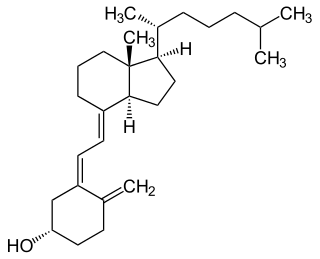
Cholecalciferol, also known as vitamin D3 and colecalciferol, is a type of vitamin D that is made by the skin when exposed to UV-B light; it is found in some foods and can be taken as a dietary supplement.

Ergocalciferol, also known as vitamin D2 and nonspecifically calciferol, is a type of vitamin D found in food and used as a dietary supplement. As a supplement it is used to prevent and treat vitamin D deficiency. This includes vitamin D deficiency due to poor absorption by the intestines or liver disease. It may also be used for low blood calcium due to hypoparathyroidism. It is used by mouth or injection into a muscle.

Sunless tanning, also known as UV filled tanning, self tanning, spray tanning, or fake tanning, refers to the effect of a suntan without exposure to the Sun. Sunless tanning involves the use of oral agents (carotenids), or creams, lotions or sprays applied to the skin. Skin-applied products may be skin-reactive agents or temporary bronzers (colorants).
Ecamsule is an organic compound which is added to many sunscreens to filter out UVA rays. It is a benzylidene camphor derivative, many of which are known for their excellent photostability.
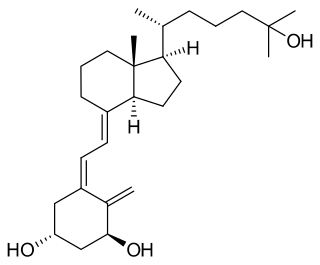
Calcitriol is the active form of vitamin D, normally made in the kidney. It is also known as 1,25-dihydroxycholecalciferol. It is a hormone which binds to and activates the vitamin D receptor in the nucleus of the cell, which then increases the expression of many genes. Calcitriol increases blood calcium (Ca2+) mainly by increasing the uptake of calcium from the intestines.

Avobenzone is an organic molecule and an oil-soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays.

Octyl methoxycinnamate or ethylhexyl methoxycinnamate (INCI) or octinoxate (USAN), trade names Eusolex 2292 and Uvinul MC80, is an organic compound that is an ingredient in some sunscreens and lip balms. It is an ester formed from methoxycinnamic acid and 2-ethylhexanol. It is a liquid that is insoluble in water.

Vitamin D toxicity, or hypervitaminosis D is the toxic state of an excess of vitamin D. The normal range for blood concentration in adults is 20 to 50 nanograms per milliliter (ng/mL).
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative stress caused by excess or fluctuating light conditions. Humans and other animals have also developed photoprotective mechanisms to avoid UV photodamage to the skin, prevent DNA damage, and minimize the downstream effects of oxidative stress.

Calcifediol, also known as calcidiol, 25-hydroxycholecalciferol, or 25-hydroxyvitamin D3 (abbreviated 25(OH)D3), is a form of vitamin D produced in the liver by hydroxylation of vitamin D3 (cholecalciferol) by the enzyme vitamin D 25-hydroxylase. Calcifediol can be further hydroxylated by the enzyme 25(OH)D-1α-hydroxylase, primarily in the kidney, to form calcitriol (1,25-(OH)2D3), which is the active hormonal form of vitamin D.
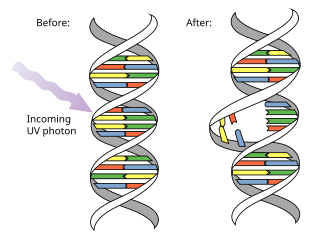
Pyrimidine dimers represent molecular lesions originating from thymine or cytosine bases within DNA, resulting from photochemical reactions. These lesions, commonly linked to direct DNA damage, are induced by ultraviolet light (UV), particularly UVC, result in the formation of covalent bonds between adjacent nitrogenous bases along the nucleotide chain near their carbon–carbon double bonds, the photo-coupled dimers are fluorescent. Such dimerization, which can also occur in double-stranded RNA (dsRNA) involving uracil or cytosine, leads to the creation of cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts. These pre-mutagenic lesions modify the DNA helix structure, resulting in abnormal non-canonical base pairing and, consequently, adjacent thymines or cytosines in DNA will form a cyclobutane ring when joined together and cause a distortion in the DNA. This distortion prevents DNA replication and transcription mechanisms beyond the dimerization site.

24,25-Dihydroxycholecalciferol, also known as 24,25-dihydroxyvitamin D3 and (24R)-hydroxycalcidiol (abbreviated as 24(R),25-(OH)2D3), is a compound which is closely related to 1,25-dihydroxyvitamin D3, the active form of vitamin D3. Like vitamin D3 itself and calcifediol (25-hydroxyvitamin D3), it is inactive as a hormone both in vitro and in vivo. It was first identified in 1972 in the laboratory of Hector DeLuca and Michael F. Holick.

Vitamin D deficiency or hypovitaminosis D is a vitamin D level that is below normal. It most commonly occurs in people when they have inadequate exposure to sunlight, particularly sunlight with adequate ultraviolet B rays (UVB). Vitamin D deficiency can also be caused by inadequate nutritional intake of vitamin D; disorders that limit vitamin D absorption; and disorders that impair the conversion of vitamin D to active metabolites, including certain liver, kidney, and hereditary disorders. Deficiency impairs bone mineralization, leading to bone-softening diseases, such as rickets in children. It can also worsen osteomalacia and osteoporosis in adults, increasing the risk of bone fractures. Muscle weakness is also a common symptom of vitamin D deficiency, further increasing the risk of fall and bone fractures in adults. Vitamin D deficiency is associated with the development of schizophrenia.

Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and for many other biological effects. In humans, the most important compounds in this group are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol).

Exposing skin to the ultraviolet radiation in sunlight has both positive and negative health effects. On the positive side, exposure allows for the synthesis of vitamin D3. Vitamin D has been suggested as having a wide range of positive health effects, which include strengthening bones and possibly inhibiting the growth of some cancers. A dietary supplement can also supply vitamin D, but there are also benefits to exposure not obtainable through Vitamin D supplementation. Long-term sun exposure is associated with reduced all-cause mortality and reduced mortality risk from cardiovascular disease (CVD), some forms of cancer, and non-CVD/noncancer related disease, with indications in these studies that Vitamin D is not the mediator. Supplementation offers limited bioavailability and no synthesis of subdermal nitric oxide. UV exposure also has positive effects for endorphin levels, and possibly for protection against multiple sclerosis. Abundant visible light to the eyes gives health benefits through its association with the timing of melatonin synthesis, maintenance of normal and robust circadian rhythms, and reduced risk of seasonal affective disorder.
Mycosporine-like amino acids (MAAs) are small secondary metabolites produced by organisms that live in environments with high volumes of sunlight, usually marine environments. The exact number of compounds within this class of natural products is yet to be determined, since they have only relatively recently been discovered and novel molecular species are constantly being discovered; however, to date their number is around 30. They are commonly described as “microbial sunscreens” although their function is believed not to be limited to sun protection. MAAs represent high potential in cosmetics, and biotechnological applications. Indeed, their UV-absorbing properties would allow to create products derived from natural photoprotectors, potentially harmless to the environment and efficient against UV damage.

Michael F. Holick is an American adult endocrinologist, specializing in vitamin D, such as the identification of both calcidiol, the major circulating form of vitamin D, and calcitriol, the active form of vitamin D. His work has been the basis for diagnostic tests and therapies for vitamin D-related diseases. He is a professor of medicine at the Boston University Medical Center and editor-in-chief of the journal Clinical Laboratory.


















