| |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌkoʊləkælˈsɪfərɒl/ |
| Other names | vitamin D3 |
| AHFS/Drugs.com | Professional Drug Facts |
| License data | |
| Routes of administration | By mouth, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.612 |
| Chemical and physical data | |
| Formula | C27H44O |
| Molar mass | 384.648 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 83 to 86 °C (181 to 187 °F) |
| Boiling point | 496.4 °C (925.5 °F) |
| Solubility in water | Practically insoluble in water, freely soluble in ethanol, methanol and some other organic solvents. Slightly soluble in vegetable oils. |
| |
| |
Cholecalciferol, also known as vitamin D3, colecalciferol or calciol, is a skin-made vitamin D that is found in certain foods and used as a dietary supplement. [3] It was first described in 1936, [4] and is on the World Health Organization's List of Essential Medicines. [5] In 2023, it was the 68th most commonly prescribed medication in the United States, with more than 9 million prescriptions, [6] [7] and is available as a generic medication. [8] [9] [10]
Contents
- Medical uses
- Vitamin D deficiency
- Other diseases
- Biochemistry
- Structure
- Mechanism of action
- Biosynthesis
- Industrial production
- Stability
- Pesticide
- See also
- References
- External links
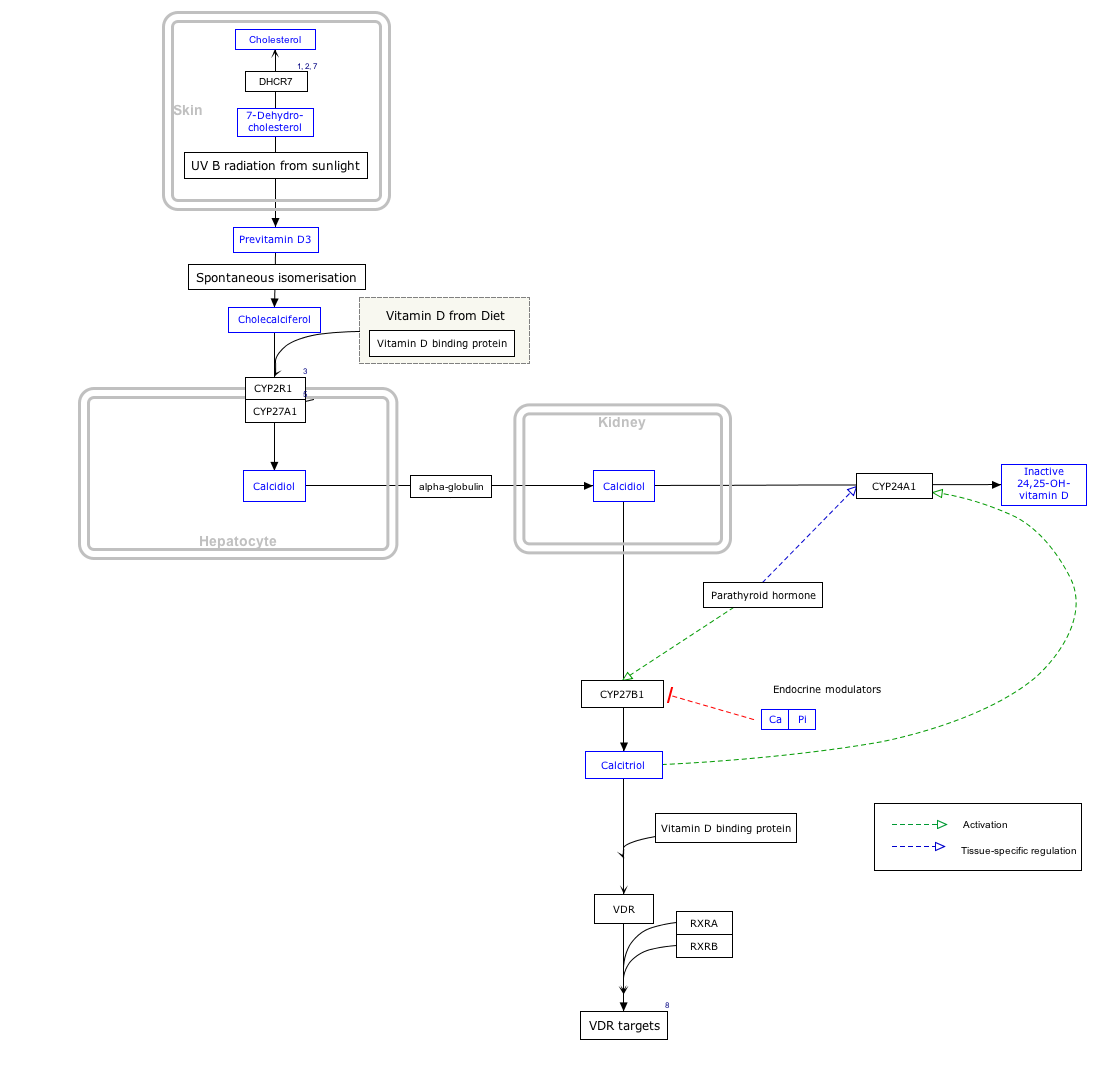
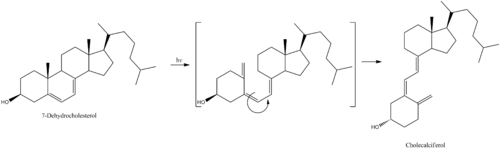
It is synthesised in the skin following sunlight exposure. [11] It is then converted in the liver to calcifediol (25-hydroxycholecalciferol D), which is further converted in the kidney to calcitriol (1,25-dihydroxycholecalciferol D). [11] One of calcitriol's most important functions is to promote calcium uptake by the intestines. [12] Cholecalciferol is present in food such as fatty fish, beef liver, eggs, and cheese. [13] [14] In some countries, cholecalciferol is also added to products like plants, cow milk, fruit juice, yogurt, and margarine. [13] [14]
Cholecalciferol can be taken orally as a dietary supplement to prevent vitamin D deficiency or as a medication to treat associated diseases, including rickets. [15] [16] It is also used in the management of familial hypophosphatemia, hypoparathyroidism that is causing low blood calcium, and Fanconi syndrome. [16] [8] Vitamin-D supplements may not be effective in people with severe kidney disease. [17] [8] Excessive doses in humans can result in vomiting, constipation, muscle weakness, and confusion. [12] Other risks include kidney stones. [17] Doses greater than 40000 IU (1000 μg) per day are generally required before high blood calcium occurs. [18] Normal doses, 800–2000 IU per day, are safe in pregnancy. [12]