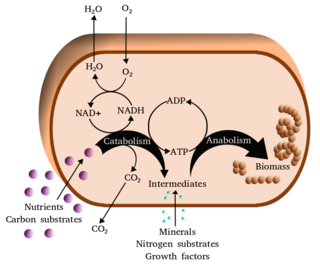
Metabolism is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks of proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary metabolism.

A steroid is an organic compound with four fused rings arranged in a specific molecular configuration.

Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. In plants, terpenes and terpenoids are important mediators of ecological interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control.

The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones.

Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the reduced form, whereas NADP+ is the oxidized form. NADP+ is used by all forms of cellular life.
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds. The study of drug metabolism is called pharmacokinetics.
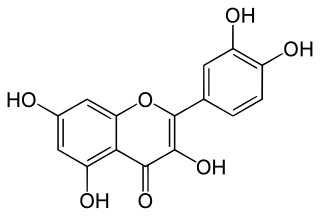
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.

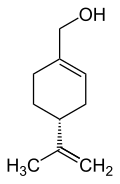
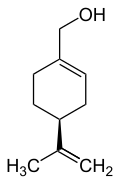
Limonene is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the volatile oil of citrus fruit peels. The (+)-isomer, occurring more commonly in nature as the fragrance of oranges, is a flavoring agent in food manufacturing. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. The less common (-)-isomer has a piny, turpentine-like odor, and is found in the edible parts of such plants as caraway, dill, and bergamot orange plants.

Methoxsalen sold under the brand name Oxsoralen among others, is a medication used to treat psoriasis, eczema, vitiligo, and some cutaneous lymphomas in conjunction with exposing the skin to ultraviolet (UVA) light from lamps or sunlight. Methoxsalen modifies the way skin cells receive the UVA radiation, allegedly clearing up the disease. Levels of individual patient PUVA exposure were originally determined using the Fitzpatrick scale. The scale was developed after patients demonstrated symptoms of phototoxicity after oral ingestion of methoxsalen followed by PUVA therapy. Chemically, methoxsalen belongs to a class of organic natural molecules known as furanocoumarins. They consist of coumarin annulated with furan. It can also be injected and used topically.

Isopentenyl pyrophosphate is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway and in the non-mevalonate MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids.
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries.

β-Hydroxy β-methylglutaryl-CoA (HMG-CoA), also known as 3-hydroxy-3-methylglutaryl coenzyme A, is an intermediate in the mevalonate and ketogenesis pathways. It is formed from acetyl CoA and acetoacetyl CoA by HMG-CoA synthase. The research of Minor J. Coon and Bimal Kumar Bachhawat in the 1950s at University of Illinois led to its discovery.

Cholesterol 7 alpha-hydroxylase also known as cholesterol 7-alpha-monooxygenase or cytochrome P450 7A1 (CYP7A1) is an enzyme that in humans is encoded by the CYP7A1 gene which has an important role in cholesterol metabolism. It is a cytochrome P450 enzyme, which belongs to the oxidoreductase class, and converts cholesterol to 7-alpha-hydroxycholesterol, the first and rate limiting step in bile acid synthesis.
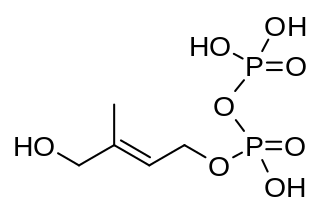
(E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP or HMB-PP) is an intermediate of the MEP pathway (non-mevalonate pathway) of isoprenoid biosynthesis. The enzyme HMB-PP synthase (GcpE, IspG) catalyzes the conversion of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MEcPP) into HMB-PP. HMB-PP is then converted further to isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) by HMB-PP reductase (LytB, IspH).
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP.
In enzymology, a (S)-limonene 7-monooxygenase (EC 1.14.13.49) is an enzyme that catalyzes the chemical reaction

Camptothecin (CPT) is a topoisomerase inhibitor. It was discovered in 1966 by M. E. Wall and M. C. Wani in systematic screening of natural products for anticancer drugs. It was isolated from the bark and stem of Camptotheca acuminata, a tree native to China used in traditional Chinese medicine. It has been used clinically in China for the treatment of gastrointestinal tumors. CPT showed anticancer activity in preliminary clinical trials, especially against breast, ovarian, colon, lung, and stomach cancers. However, it has low solubility and adverse effects have been reported when used therapeutically, so synthetic and medicinal chemists have developed numerous syntheses of camptothecin and various derivatives to increase the benefits of the chemical, with good results. Four CPT analogues have been approved and are used in cancer chemotherapy today: topotecan, irinotecan, belotecan, and trastuzumab deruxtecan. Camptothecin has also been found in other plants including Chonemorpha fragrans.

Diphosphomevalonate decarboxylase (EC 4.1.1.33), most commonly referred to in scientific literature as mevalonate diphosphate decarboxylase, is an enzyme that catalyzes the chemical reaction

(+)-Costunolide is a naturally occurring sesquiterpene lactone, first isolated in Saussurea costus roots in 1960. It is also found in lettuce.

Harpagoside is a natural product found in the plant Harpagophytum procumbens, also known as devil's claw. It is the active chemical constituent responsible for the medicinal properties of the plant, which have been used for centuries by the Khoisan people of southern Africa to treat diverse health disorders, including fever, diabetes, hypertension, and various blood related diseases.