 | |
 | |
| Names | |
|---|---|
| IUPAC name Abieta-7,13-dien-18-oic acid | |
| Systematic IUPAC name (1R,4aR,4bR,10aR)-1,4a-Dimethyl-7-(propan-2-yl)-1,2,3,4,4a,4b,5,6,10,10a-decahydrophenanthrene-1-carboxylic acid | |
| Other names Abietinic acid; Sylvic acid | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.436 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C20H30O2 | |
| Molar mass | 302.458 g·mol−1 |
| Appearance | Colorless solid |
| Density | 1.06 g/mL |
| Melting point | 172–175 °C (342–347 °F; 445–448 K) [1] |
| Insoluble [1] | |
| Solubility in other solvents | Very soluble in acetone, petroleum ether, Et2O, and ethanol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Irritant |
| GHS labelling: | |
 | |
| Warning | |
| H317 | |
| P261, P272, P280, P302+P352, P321, P333+P313, P363, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
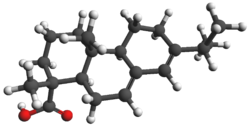
Abietic acid (also known as abietinic acid or sylvic acid) is a diterpenoid found in coniferous trees. It is supposed to exist to defend the host plant from insect attack or various wounds. Chemically, it is a complicated molecule featuring two alkene groups and a carboxylic acid within a chiral tricyclic framework. As the major component of rosin, it is commercially important. Historically speaking, it was a major component of naval stores. It is the most common of the resin acids. Another common resin acid is pimaric acid, which converts to abietic acid upon heating.


