
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The terms atomic orbital and molecular orbital were introduced by Robert S. Mulliken in 1932 to mean one-electron orbital wave functions. At an elementary level, they are used to describe the region of space in which a function has a significant amplitude.

In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele.
A halogen addition reaction is a simple organic reaction where a halogen molecule is added to the carbon–carbon double bond of an alkene functional group.
In organic chemistry, the oxymercuration reaction is an electrophilic addition reaction that transforms an alkene into a neutral alcohol. In oxymercuration, the alkene reacts with mercuric acetate in aqueous solution to yield the addition of an acetoxymercury group and a hydroxy group across the double bond. Carbocations are not formed in this process and thus rearrangements are not observed. The reaction follows Markovnikov's rule and it is an anti addition.

In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap in a continuous cycle at the transition state. Pericyclic reactions stand in contrast to linear reactions, encompassing most organic transformations and proceeding through an acyclic transition state, on the one hand and coarctate reactions, which proceed through a doubly cyclic, concerted transition state on the other hand. Pericyclic reactions are usually rearrangement or addition reactions. The major classes of pericyclic reactions are given in the table below. Ene reactions and cheletropic reactions are often classed as group transfer reactions and cycloadditions/cycloeliminations, respectively, while dyotropic reactions and group transfer reactions are rarely encountered.
In organic chemistry, an electrocyclic reaction is a type of pericyclic, rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice versa. These reactions are usually categorized by the following criteria:
In organic chemistry, a sigmatropic reaction is a pericyclic reaction wherein the net result is one sigma bond (σ-bond) is changed to another σ-bond in an intramolecular reaction. In this type of rearrangement reaction, a substituent moves from one part of a π-system to another part with simultaneous rearrangement of the π-system. True sigmatropic reactions are usually uncatalyzed, although Lewis acid catalysis is possible. Sigmatropic reactions often have transition-metal catalysts that form intermediates in analogous reactions. The most well-known of the sigmatropic rearrangements are the [3,3] Cope rearrangement, Claisen rearrangement, Carroll rearrangement, and the Fischer indole synthesis.
The 1,3-dipolar cycloaddition is a chemical reaction between a 1,3-dipole and a dipolarophile to form a five-membered ring. The earliest 1,3-dipolar cycloadditions were described in the late 19th century to the early 20th century, following the discovery of 1,3-dipoles. Mechanistic investigation and synthetic application were established in the 1960s, primarily through the work of Rolf Huisgen. Hence, the reaction is sometimes referred to as the Huisgen cycloaddition. 1,3-dipolar cycloaddition is an important route to the regio- and stereoselective synthesis of five-membered heterocycles and their ring-opened acyclic derivatives. The dipolarophile is typically an alkene or alkyne, but can be other pi systems. When the dipolarophile is an alkyne, aromatic rings are generally produced.
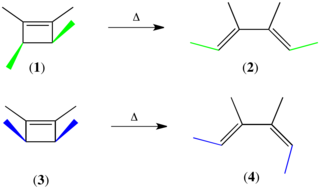
The Woodward–Hoffmann rules are a set of rules devised by Robert Burns Woodward and Roald Hoffmann to rationalize or predict certain aspects of the stereochemistry and activation energy of pericyclic reactions, an important class of reactions in organic chemistry. The rules originate in certain symmetries of the molecule's orbital structure that any molecular Hamiltonian conserves. Consequently, any symmetry-violating reaction must couple extensively to the environment; this imposes an energy barrier on its occurrence, and such reactions are called symmetry-forbidden. Their opposites are symmetry-allowed.

In organic chemistry, antarafacial and suprafacial (s) are two topological concepts in organic chemistry describing the relationship between two simultaneous chemical bond making and/or breaking processes in or around a reaction center. The reaction center can be a p- or spn-orbital, a conjugated system (π) or even a sigma bond (σ).
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane. MO diagrams can explain why some molecules exist and others do not. They can also predict bond strength, as well as the electronic transitions that can take place.
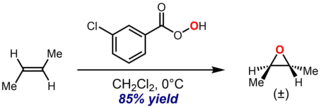
The Prilezhaev reaction, also known as the Prileschajew reaction or Prilezhaev epoxidation, is the chemical reaction of an alkene with a peroxy acid to form epoxides. It is named after Nikolai Prilezhaev, who first reported this reaction in 1909. A widely used peroxy acid for this reaction is meta-chloroperoxybenzoic acid (m-CPBA), due to its stability and good solubility in most organic solvents. The reaction is performed in inert solvents (C6H14, C6H6, CH2Cl2, CHCl3, CCl4) between -10 and 60 °C with the yield of 60-80%.
A non-bonding orbital, also known as non-bonding molecular orbital (NBMO), is a molecular orbital whose occupation by electrons neither increases nor decreases the bond order between the involved atoms. Non-bonding orbitals are often designated by the letter n in molecular orbital diagrams and electron transition notations. Non-bonding orbitals are the equivalent in molecular orbital theory of the lone pairs in Lewis structures. The energy level of a non-bonding orbital is typically in between the lower energy of a valence shell bonding orbital and the higher energy of a corresponding antibonding orbital. As such, a non-bonding orbital with electrons would commonly be a HOMO.
In chemistry, frontier molecular orbital theory is an application of molecular orbital theory describing HOMO–LUMO interactions.

Walsh diagrams, often called angular coordinate diagrams or correlation diagrams, are representations of calculated orbital binding energies of a molecule versus a distortion coordinate, used for making quick predictions about the geometries of small molecules. By plotting the change in molecular orbital levels of a molecule as a function of geometrical change, Walsh diagrams explain why molecules are more stable in certain spatial configurations.
In chemistry, the Möbius–Hückel treatment is a methodology used to predict whether a reaction is allowed or forbidden. It is often used along with the Woodward–Hoffmann approach. The description in this article uses the plus and minus sign notation for parity as shorthand while proceeding around a cycle of orbitals in a molecule or system, while the Woodward–Hoffmann methodology uses a large number of rules with the same consequences.
The inverse electron demand Diels–Alder reaction, or DAINV or IEDDA is an organic chemical reaction, in which two new chemical bonds and a six-membered ring are formed. It is related to the Diels–Alder reaction, but unlike the Diels–Alder reaction, the DAINV is a cycloaddition between an electron-rich dienophile and an electron-poor diene. During a DAINV reaction, three pi-bonds are broken, and two sigma bonds and one new pi-bond are formed. A prototypical DAINV reaction is shown on the right.
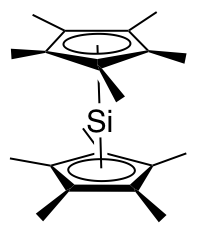
Decamethylsilicocene, (C5Me5)2Si, is a group 14 sandwich compound. It is an example of a main-group cyclopentadienyl complex; these molecules are related to metallocenes but contain p-block elements as the central atom. It is a colorless, air sensitive solid that sublimes under vacuum.
Second-order Jahn-Teller distortion is a singular, general, and powerful approach rigorously based in first-principle vibronic coupling interactions. It enables prediction and explication of molecular geometries that are not necessarily satisfactorily or even correctly explained by semi-empirical theories such as Walsh diagrams, atomic state hybridization, valence shell electron pair repulsion (VSEPR), softness-hardness-based models, aromaticity and antiaromaticity, hyperconjugation, etc.









