
In polymer chemistry and materials science, a resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses on naturally occurring resins.

Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine.

The Pinaceae, or pine family, are conifer trees or shrubs, including many of the well-known conifers of commercial importance such as cedars, firs, hemlocks, larches, pines and spruces. The family is included in the order Pinales, formerly known as Coniferales. Pinaceae are supported as monophyletic by their protein-type sieve cell plastids, pattern of proembryogeny, and lack of bioflavonoids. They are the largest extant conifer family in species diversity, with between 220 and 250 species in 11 genera, and the second-largest in geographical range, found in most of the Northern Hemisphere, with the majority of the species in temperate climates, but ranging from subarctic to tropical. The family often forms the dominant component of boreal, coastal, and montane forests. One species, Pinus merkusii, grows just south of the equator in Southeast Asia. Major centres of diversity are found in the mountains of southwest China, Mexico, central Japan, and California.

Abietic acid is an organic compound that occurs widely in trees. It is the primary component of resin acid, is the primary irritant in pine wood and resin, isolated from rosin and is the most abundant of several closely related organic acids that constitute most of rosin, the solid portion of the oleoresin of coniferous trees. Its ester or salt is called an abietate.

Ginkgolides are biologically active terpenic lactones present in Ginkgo biloba. They are diterpenoids with 20-carbon skeletons, which are biosynthesized from geranylgeranyl pyrophosphate.
Diterpenes are a class of terpenes composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and anti-inflammatory.
Geranylgeranyl pyrophosphate is an intermediate in the biosynthesis of diterpenes and diterpenoids. It is also the precursor to carotenoids, gibberellins, tocopherols, and chlorophylls.
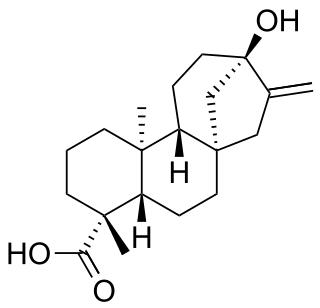
Steviol is a diterpene first isolated from the plant Stevia rebaudiana in 1931. Its chemical structure was not fully elucidated until 1960.

Ferruginol is a natural phenol with a terpenoid substructure. Specifically, it is a diterpene of the abietane chemical class, meaning it is characterized by three fused six-membered rings and alkyl functional groups. Ferruginol was first identified in 1939 by Brandt and Neubauer as the main component in the resin of the Miro tree and has since been isolated from other conifer species in the families Cupressaceae and Podocarpaceae. As a biomarker, the presence of ferruginol in fossils, mainly resin, is used to describe the density of these conifers in that particular biosphere throughout time.
The enzyme abieta-7,13-diene synthase catalyzes the chemical reaction
The enzyme taxadiene synthase catalyzes the chemical reaction

Juvabione, historically known as the paper factor, is the methyl ester of todomatuic acid. Both are sesquiterpenes (C15) found in the wood of true firs of the genus Abies. They occur naturally as part of a mixture of sesquiterpenes based upon the bisabolane scaffold. Sesquiterpenes of this family are known as insect juvenile hormone analogues (IJHA) because of their ability to mimic juvenile activity in order to stifle insect reproduction and growth. These compounds play important roles in conifers as the second line of defense against insect induced trauma and fungal pathogens.

Taxodone is a naturally occurring diterpenoid found in Taxodium distichum, Rosmarinus officinalis (rosemary), several salvia species and other plants, along with its oxidized rearrangement product, taxodione. Taxodone and taxodione exhibit anticancer, antibacterial, antioxidant, antifungal, insecticide, and antifeedant activities.
In molecular biology, this protein domain belongs to the terpene synthase family (TPS). Its role is to synthesize terpenes, which are part of primary metabolism, such as sterols and carotene, and also part of the secondary metabolism. This entry will focus on the C terminal domain of the TPS protein.
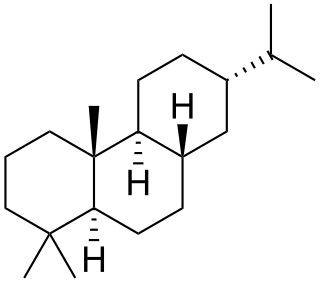
Abietane is a diterpene that forms the structural basis for a variety of natural chemical compounds such as abietic acid, carnosic acid, and ferruginol which are collectively known as abietanes or abietane diterpenes.
Abieta-7,13-dien-18-al dehydrogenase (EC 1.2.1.74, abietadienal dehydrogenase (ambiguous)) is an enzyme with systematic name abieta-7,13-dien-18-al:NAD+ oxidoreductase. This enzyme catalyses the following chemical reaction
Abieta-7,13-diene hydroxylase (EC 1.14.13.108) is an enzyme with systematic name abieta-7,13-diene,NADPH:oxygen oxidoreductase (18-hydroxylating). This enzyme catalyses the following chemical reaction
Abieta-7,13-dien-18-ol hydroxylase (EC 1.14.13.109, CYP720B1, PTAO) is an enzyme with systematic name abieta-7,13-dien-18-ol,NADPH:oxygen oxidoreductase (18-hydroxylating). This enzyme catalyses the following chemical reaction
Levopimaradiene synthase is an enzyme with systematic name (+)-copalyl-diphosphate diphosphate-lyase . This enzyme catalyses the following chemical reaction
Neoabietadiene synthase is an enzyme with systematic name (+)-copaly-diphosphate diphosphate-lyase . This enzyme catalyses the following chemical reaction:












