 | |
| Names | |
|---|---|
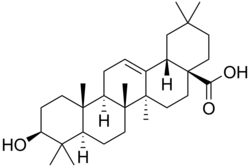
| IUPAC name 3β-Hydroxyolean-12-en-28-oic acid | |
| Systematic IUPAC name (4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-Hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid | |
| Other names Oleanic acid | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.347 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C30H48O3 | |
| Molar mass | 456.711 g·mol−1 |
| Appearance | White |
| Melting point | >300 °C (572 °F; 573 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins. [2]
