| |||
| Names | |||
|---|---|---|---|
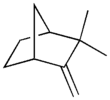
| Preferred IUPAC name 2,2-Dimethyl-3-methylidenebicyclo[2.2.1]heptane | |||
| Other names 2,2-Dimethyl-3-methanylidenebicyclo[2.2.1]heptane 2,2-Dimethyl-3-methylenebicyclo[2.2.1]heptane | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.123 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 2319 1325 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C10H16 | |||
| Molar mass | 136.238 g·mol−1 | ||
| Appearance | White or colorless solid [1] | ||
| Density | 0.842 g/cm3 [1] | ||
| Melting point | 51 to 52 °C (124 to 126 °F; 324 to 325 K) [1] | ||
| Boiling point | 159 °C (318 °F; 432 K) [1] | ||
| Practically insoluble [1] | |||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Warning | |||
| H226, H228, H319, H410 | |||
| P210, P233, P240, P241, P242, P243, P264, P273, P280, P303+P361+P353, P305+P351+P338, P337+P313, P370+P378, P391, P403+P235, P501 | |||
| Flash point | 40 °C (104 °F; 313 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As with other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell. [2] It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil, neroli, ginger oil, valerian, and mango. [3] It is produced industrially by isomerization of the more common alpha-pinene using a solid acid catalyst such as titanium dioxide. [4]
Camphene is used in the preparation of fragrances and as a food additive for flavoring. These include isobornyl acetate.