
Glycogen phosphorylase is one of the phosphorylase enzymes. Glycogen phosphorylase catalyzes the rate-limiting step in glycogenolysis in animals by releasing glucose-1-phosphate from the terminal alpha-1,4-glycosidic bond. Glycogen phosphorylase is also studied as a model protein regulated by both reversible phosphorylation and allosteric effects.
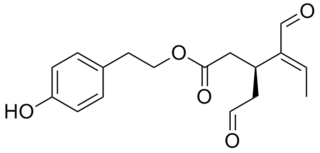
Oleocanthal is a phenylethanoid, or a type of natural phenolic compound found in extra-virgin olive oil. It appears to be responsible for the burning sensation that occurs in the back of the throat when consuming such oil. Oleocanthal is a tyrosol ester and its chemical structure is related to oleuropein, also found in olive oil.
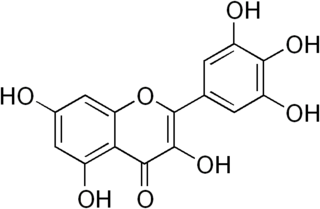
Myricetin is a member of the flavonoid class of polyphenolic compounds, with antioxidant properties. Common dietary sources include vegetables, fruits, nuts, berries, tea, and red wine.
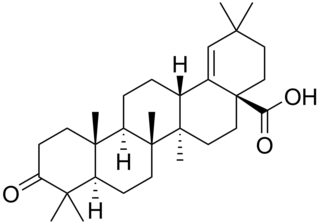
Moronic acid is a natural triterpene. Moronic acid can be extracted from Rhus javanica, a sumac plant traditionally believed to hold medicinal applications. The molecule has also been extracted from mistletoe.

Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids.

Boswellic acids are a series of pentacyclic terpenoid molecules that are produced by plants in the genus Boswellia. Like many other terpenes, boswellic acids appear in the resin of the plant that exudes them; it is estimated that they make up 30% of the resin of Boswellia serrata. While boswellic acids are a major component of the resin, the steam or hydro distilled frankincense essential oil does not contain any boswellic acid as these components are non-volatile and too large to come over in the steam distillation process.
Chemokine ligand 1 (CCL1) is also known as small inducible cytokine A1 and I-309 in humans. CCL1 is a small glycoprotein that belongs to the CC chemokine family.

Mitraphylline, an oxindole derivative, is an active alkaloid in the leaves of the tree Mitragyna speciosa, commonly known as kratom. As a non-narcotic constituent, it also occurs to a significant amount in the bark of Uncaria tomentosa along with a number of isomeric alkaloids.
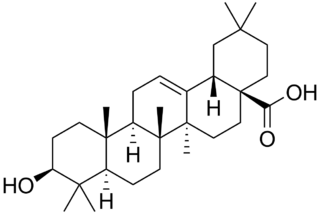
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins.

A depside is a type of polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester group. Depsides are most often found in lichens, but have also been isolated from higher plants, including species of the Ericaceae, Lamiaceae, Papaveraceae and Myrtaceae.

Protein phosphatase 1 (PP1) belongs to a certain class of phosphatases known as protein serine/threonine phosphatases. This type of phosphatase includes metal-dependent protein phosphatases (PPMs) and aspartate-based phosphatases. PP1 has been found to be important in the control of glycogen metabolism, muscle contraction, cell progression, neuronal activities, splicing of RNA, mitosis, cell division, apoptosis, protein synthesis, and regulation of membrane receptors and channels.

Croton lechleri is a species of flowering plant in the spurge family, Euphorbiaceae, that is native to northwestern South America. It is commonly known as sangre de grado, sangre de drago or sangre de grada. They refer to this tree's thick red latex.

The amyrins are three closely related natural chemical compounds of the triterpene class. They are designated α-amyrin (ursane skeleton), β-amyrin (oleanane skeleton) and δ-amyrin. Each is a pentacyclic triterpenol with the chemical formula C30H50O. They are widely distributed in nature and have been isolated from a variety of plant sources such as epicuticular wax. In plant biosynthesis, α-amyrin is the precursor of ursolic acid and β-amyrin is the precursor of oleanolic acid. All three amyrins occur in the surface wax of tomato fruit. α-Amyrin is found in dandelion coffee.

Cucurbitacin E is a biochemical compound from the family of cucurbitacins. These are found in plants which are member of the family Cucurbitaceae, most of them coming from traditional Chinese medicinal plants, but also in other plants such as pumpkins and gourds.
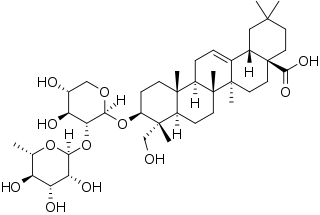
α-Hederin (alpha-hederin) is a water-soluble pentacyclic triterpenoid saponin found in the seeds of Nigella sativa and leaves of Hedera helix.

Nyasol, also known as cis-hinokiresinol or as (Z)-hinokiresinol, is a lignan that is found in Anemarrhena asphodeloides. It has estrogenic activity, acting as a selective agonist of the ERβ, and hence is a phytoestrogen. In addition, (-)-nyasol has been found to inhibit the production of eicosanoids and nitric oxide in vitro and shows anti-inflammatory effects.
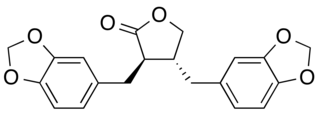
Hinokinin is a dibenzylbutyrolactone lignan, derived from various species of plants. It is a potential antichagonistic agent and has shown to possess neuroprotective effects as well. It is also found to have anti-inflammatory, anti-cancer, antiviral and antifungal properties.

Ferula narthex is a species of plant native to Afghanistan, Tajikistan, northern Pakistan and Kashmir. Although it is often listed as the source of asafoetida, one report stated that its essential oil lacked sulfur-containing compounds which are characteristic of asafoetida.
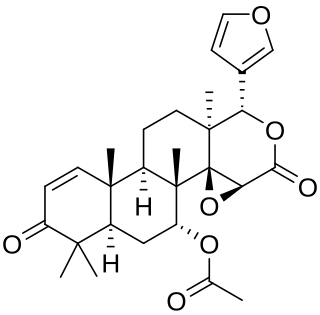
Gedunin is a pentacyclic triterpenoid with the molecular formula C28H34 O7. It is most notably found in Azadirachta indica, but is a constituent of several other plants. Gedunin shows therapeutic potential in the treatment of leukemia, and Parkinson's disease.

Cussonia arborea is a deciduous small to medium sized tree within the family Araliaceae. Extracts of the species are widely used in traditional medicine to treat a variety of ailments.



















