
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.
Butene, also known as butylene, is an alkene with the formula C4H8. The word butene may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for viable extraction. Butene is therefore obtained by catalytic cracking of long-chain hydrocarbons left during refining of crude oil. Cracking produces a mixture of products, and the butene is extracted from this by fractional distillation.

Gas chromatography–mass spectrometry (GC–MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC–MS include drug detection, fire investigation, environmental analysis, explosives investigation, food and flavor analysis, and identification of unknown samples, including that of material samples obtained from planet Mars during probe missions as early as the 1970s. GC–MS can also be used in airport security to detect substances in luggage or on human beings. Additionally, it can identify trace elements in materials that were previously thought to have disintegrated beyond identification. Like liquid chromatography–mass spectrometry, it allows analysis and detection even of tiny amounts of a substance.

Brassicasterol is a 28-carbon sterol synthesised by several unicellular algae (phytoplankton) and some terrestrial plants, like rape. This compound has frequently been used as a biomarker for the presence of (marine) algal matter in the environment, and is one of the ingredients in stigmasterol-rich plant sterols. There is some evidence to suggest that it may also be a relevant additional biomarker in Alzheimer's disease.

Cholestane is a saturated tetracyclic triterpene. This 27-carbon biomarker is produced by diagenesis of cholesterol and is one of the most abundant biomarkers in the rock record. Presence of cholestane, its derivatives and related chemical compounds in environmental samples is commonly interpreted as an indicator of animal life and/or traces of O2, as animals are known for exclusively producing cholesterol, and thus has been used to draw evolutionary relationships between ancient organisms of unknown phylogenetic origin and modern metazoan taxa. Cholesterol is made in low abundance by other organisms (e.g., rhodophytes, land plants), but because these other organisms produce a variety of sterols it cannot be used as a conclusive indicator of any one taxon. It is often found in analysis of organic compounds in petroleum.

Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids.
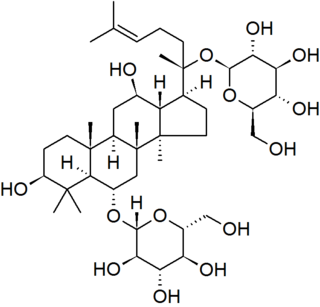
Ginsenosides or panaxosides are a class of natural product steroid glycosides and triterpene saponins. Compounds in this family are found almost exclusively in the plant genus Panax (ginseng), which has a long history of use in traditional medicine that has led to the study of pharmacological effects of ginseng compounds. As a class, ginsenosides exhibit a large variety of subtle and difficult-to-characterize biological effects when studied in isolation.
Abietane is a diterpene that forms the structural basis for a variety of natural chemical compounds such as abietic acid, carnosic acid, and ferruginol which are collectively known as abietanes or abietane diterpenes.
Bacteriohopanepolyols (BHPs), bacteriohopanoids, or bacterial pentacyclic triterpenoids are commonly found in the lipid cell membranes of bacteria. BHPs are frequently used as biomarkers in sedimentary rocks and can provide paleoecological information about ancient bacterial communities.
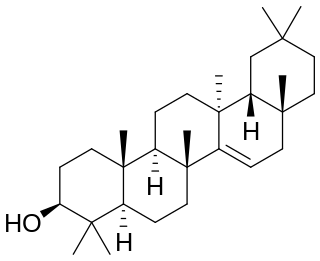
Taraxerol is a naturally-occurring pentacyclic triterpenoid. It exists in various higher plants, including Taraxacum officinale (Asteraceae), Alnus glutinosa (Betulaceae), Litsea dealbata (Lauraceae), Skimmia spp. (Rutaceae), Dorstenia spp. (Moraceae), Maytenus spp. (Celastraceae), and Alchornea latifolia (Euphobiaceae). Taraxerol was named "alnulin" when it was first isolated in 1923 from the bark of the grey alder by Zellner and Röglsperger. It also had the name "skimmiol" when Takeda and Yosiki isolated it from Skimmia (Rutaceae). A large number of medicinal plants are known to have this compound in their leaves, roots or seed oil.

24-n-Propylcholestane is a sterane biomarker molecule often found in marine source rocks. It is a C30 molecule, meaning that it is composed of thirty carbon atoms, and is one of the leading ways to distinguish a marine source rock from a terrigenous sample. It is composed of three six-carbon rings and one five-carbon ring, with two methyl groups and one eleven carbon side chain. 24-n-Propylcholestane has a molar mass of 414.76 g/mol.

Bisnorhopanes (BNH) are a group of demethylated hopanes found in oil shales across the globe and can be used for understanding depositional conditions of the source rock. The most common member, 28,30-bisnorhopane, can be found in high concentrations in petroleum source rocks, most notably the Monterey Shale, as well as in oil and tar samples. 28,30-Bisnorhopane was first identified in samples from the Monterey Shale Formation in 1985. It occurs in abundance throughout the formation and appears in stratigraphically analogous locations along the California coast. Since its identification and analysis, 28,30-bisnorhopane has been discovered in oil shales around the globe, including lacustrine and offshore deposits of Brazil, silicified shales of the Eocene in Gabon, the Kimmeridge Clay Formation in the North Sea, and in Western Australian oil shales.

24-Norcholestane, a steroid derivative, is used as a biomarker to constrain the source age of sediments and petroleum through the ratio between 24-norcholestane and 27-norcholestane, especially when used with other age diagnostic biomarkers, like oleanane. While the origins of this compound are still unknown, it is thought that they are derived from diatoms due to their identification in diatom rich sediments and environments. In addition, it was found that 24-norcholestane levels increased in correlation with diatom evolution. Another possible source of 24-norcholestane is from dinoflagellates, albeit to a much lower extent.
Chlorobactane is the diagenetic product of an aromatic carotenoid produced uniquely by green-pigmented green sulfur bacteria (GSB) in the order Chlorobiales. Observed in organic matter as far back as the Paleoproterozoic, its identity as a diagnostic biomarker has been used to interpret ancient environments.

Tetrahymanol is a gammacerane-type membrane lipid first found in the marine ciliate Tetrahymena pyriformis. It was later found in other ciliates, fungi, ferns, and bacteria. After being deposited in sediments that compress into sedimentary rocks over millions of years, tetrahymanol is dehydroxylated into gammacerane. Gammacerane has been interpreted as a proxy for ancient water column stratification.

Isoarborinol is a triterpenoid ubiquitously produced by angiosperms and is thus considered a biomarker for higher plants. Though no isoarborinol-producing microbe has been identified, isoarborinol is also considered a possible biomarker for marine bacteria, as its diagenetic end product, arborane, has been found in ancient marine sediments that predate the rise of plants. Importantly, isoarborinol may represent the phylogenetic link between hopanols and sterols.

Sugiol is a phenolic abietane derivative of ferruginol and can be used as a biomarker for specific families of conifers. The presence of sugiol can be used to identify the Cupressaceae s.1., podocarpaceae, and Araucaraiaceae families of conifers. The polar terpenoids are among the most resistant molecules to degradation besides n-alkanes and fatty acids, affording them high viability as biomarkers due to their longevity in the sedimentary record. Significant amounts of sugiol has been detected in fossil wood dated to the Eocene and Miocene periods, as well as a sample of Protopodocarpoxylon dated to the middle Jurassic.
Arborane is a class of pentacyclic triterpene consisting of organic compounds with four 6-membered rings and one 5-membered ring. Arboranes are thought to be derived from arborinols, a class of natural cyclic triterpenoids typically produced by flowering plants. Thus arboranes are used as a biomarker for angiosperms and cordaites. Arborane is a stereoisomer of a compound called fernane, the diagenetic product of fernene and fernenol. Because aborinol and fernenol have different biological sources, the ratio of arborane/fernane in a sample can be used to reconstruct a record for the relative abundances of different plants.
Biphytane (or bisphytane) is a C40 isoprenoid produced from glycerol dialkyl glycerol tetraether (GDGT) degradation. As a common lipid membrane component, biphytane is widely used as a biomarker for archaea. In particular, given its association with sites of active anaerobic oxidation of methane (AOM), it is considered a biomarker of methanotrophic archaea. It has been found in both marine and terrestrial environments.

Diplopterol is a triterpenoid molecule commonly produced by bacteria, ferns, and a few protozoans. This compound, classified as a member of the hopanoid family, is synthesized from triterpenoid precursor squalene. It is generally believed that hopanoids serve a similar function in bacteria as that of sterols in eukaryotes, which involves modulating membrane fluidity. Diplopterol serves as a useful biomarker for prokaryotic life, along with oxygen content at the time of sediment deposition.















