In polymer chemistry and materials science, a resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses mainly on naturally occurring resins.

Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.

Dammar, also called dammar gum, or damar gum, is a resin obtained from the tree family Dipterocarpaceae in India and Southeast Asia, principally those of the genera Shorea or Hopea. The resin of some species of Canarium may also called dammar. Most is produced by tapping trees; however, some is collected in fossilised form on the ground. The gum varies in colour from clear to pale yellow, while the fossilised form is grey-brown. Dammar gum is a triterpenoid resin, containing many triterpenes and their oxidation products. Many of them are low molecular weight compounds, which easily oxidizes and photoxidizes.

Resorcinol (or resorcin) is a phenolic compound. It is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or meta-isomer). Resorcinol crystallizes from benzene as colorless needles that are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide.

Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, and in the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers. Some astrobiologists suggest that it may be an alternative to water as the main solvent in other forms of life.

Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants.

Indene is an aromatic, polycyclic hydrocarbon with chemical formula C9H8. It is composed of a benzene ring fused with a cyclopentene ring. This flammable liquid is colorless although samples often are pale yellow. The principal industrial use of indene is in the production of indene/coumarone thermoplastic resins. Substituted indenes and their closely related indane derivatives are important structural motifs found in many natural products and biologically active molecules, such as sulindac.

1-Butene (IUPAC name: But-1-ene, also known as 1-butylene) is the organic compound with the formula CH3CH2CH=CH2. It is a colorless gas. But-1-ene is an alkene easily condensed to give a colorless liquid. It is classified as a linear alpha-olefin (terminal alkene). It is one of the isomers of butene (butylene). It is a precursor to diverse products.

sec-Butyl acetate, or s-butyl acetate, is an ester commonly used as a solvent in lacquers and enamels, where it is used in the production of acyclic polymers, vinyl resins, and nitrocellulose. It is a clear flammable liquid with a sweet smell.
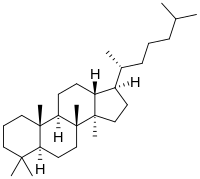
Cholane is a triterpene which can exist as either of two stereoisomers, 5α-cholane and 5β-cholane. Its name is derived from Greek: χολή (chole) meaning 'bile' in reference to its original discovery from the bile of the American bullfrog. The compound itself has no known uses. However, various functionalized analogues are produced by plants and animals, typically in the form of sterols, steroids and bile acids.

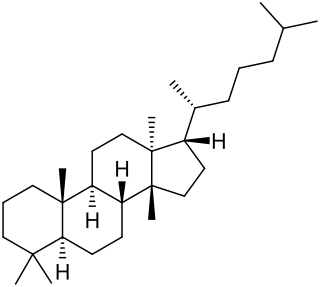
Hopane is a natural chemical compound classified as a triterpene. It forms the central core of a variety of other chemical compounds which are collectively known as hopanoids. The first compound of the hopane family to be isolated and characterised was hydroxyhopanone, found in dammar resin. The name derives from Hopea, a tree genus from which dammar is obtained.
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among all possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations.

Isobenzofuran is a bicyclic heterocycle consisting of fused cyclohexa-1,3-diene and furan rings. It is isomeric with benzofuran.

Lanostane or 4,4,14α-trimethylcholestane is a tetracyclic chemical compound with formula C
30H
54. It is a polycyclic hydrocarbon, specifically a triterpene. It is an isomer of cucurbitane.

Stigmastane or 24R-ethylcholestane is a tetracyclic triterpene, along with cholestane and ergostane, this sterane is used as a biomarker for early eukaryotes.

Protostane is a tetracyclic triterpene, its natural distribution is primarily limited to the genus Alisma. It is so named because it is considered to be the "prototype" of steroids.

Gorgostane is a steroid triterpene, its derivative distributed in corals, hence the name. Compared with other steroids, there is a cyclopropane ring in the 17C side-chain.

Campestane or 24R-methylcholestane is a tetracyclic triterpene. Its derivative campesterol (campest-5-en-3β-ol) was first isolated from the rapeseed, hence the name.
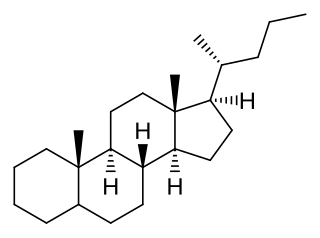
Poriferastane or 24S-ethylcholestane is a tetracyclic triterpene and the parent structure of a series of steroids, such as poriferastanol.
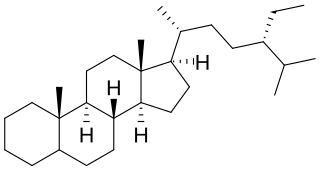
Euphane is a tetracyclic triterpene that is the 13α,14β-stereoisomer of lanostane. Its derivatives are widely distributed in many plants.