 | |
| Names | |
|---|---|
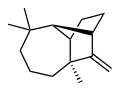
| IUPAC name (1R,2S,7S,9S)- 3,3,7-trimethyl- 8-methylenetricyclo- [5.4.0.02,9]undecane | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 5731712 2044263 4663756 | |
| ChEBI |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.812 |
| EC Number |
|
PubChem CID | |
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.36 g/mol |
| Density | 0.928 g/cm3 |
| Boiling point | 254 °C (489 °F; 527 K) (706 mm Hg) |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H304, H317, H410 | |
| P261, P272, P273, P280, P301+P310, P302+P352, P321, P331, P333+P313, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Longifolene is a common sesquiterpene. It is an oily liquid hydrocarbon found primarily in the high-boiling fraction of certain pine resins. The name is derived from that of a pine species from which the compound was isolated. [1] It is a tricyclic chiral molecule. The enantiomer commonly found in pines and other higher plants exhibits a positive optical rotation of +42.73°. The other enantiomer (optical rotation −42.73°) is found in small amounts in certain fungi and liverworts.

