Contents
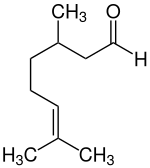 | |
 (+)-Citronellal | |
 (-)-Citronellal | |
| Names | |
|---|---|
| IUPAC name 3,7-dimethyloct-6-enal | |
| Identifiers | |
3D model (JSmol) | |
| 1209447 1720789 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.070 |
| EC Number |
|
| 1521962 | |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C10H18O | |
| Molar mass | 154.25 g/mol |
| Density | 0.855 g/cm3 |
| Boiling point | 201 to 207 °C (394 to 405 °F; 474 to 480 K) |
| Hazards | |
| GHS labelling: | |
  [2] [2] | |
| Warning | |
| H315, H317, H411 [2] | |
| P262, P273, P280, P302+P352 [2] | |
| Related compounds | |
Related alkenals | Citral |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Citronellal or rhodinal (C 10 H 18 O) is a monoterpenoid aldehyde, the main component in the mixture of terpenoid chemical compounds that give citronella oil its distinctive lemon scent.
Citronellal is a main isolate in distilled oils from the plants Cymbopogon (excepting C. citratus, culinary lemongrass), [3] lemon-scented gum, and lemon-scented teatree. The (S)-(−)-enantiomer of citronellal makes up to 80% of the oil from kaffir lime leaves and is the compound responsible for its characteristic aroma.
Citronellal has insect repellent properties, and research shows high repellent effectiveness against mosquitoes. [4] Another research shows that citronellal has strong antifungal qualities. [5]