Apoptosis is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses 50 to 70 billion cells each day due to apoptosis. For the average human child between 8 and 14 years old, each day the approximate loss is 20 to 30 billion cells.
Granzymes are serine proteases released by cytoplasmic granules within cytotoxic T cells and natural killer (NK) cells. They induce programmed cell death (apoptosis) in the target cell, thus eliminating cells that have become cancerous or are infected with viruses or bacteria. Granzymes also kill bacteria and inhibit viral replication. In NK cells and T cells, granzymes are packaged in cytotoxic granules along with perforin. Granzymes can also be detected in the rough endoplasmic reticulum, golgi complex, and the trans-golgi reticulum. The contents of the cytotoxic granules function to permit entry of the granzymes into the target cell cytosol. The granules are released into an immune synapse formed with a target cell, where perforin mediates the delivery of the granzymes into endosomes in the target cell, and finally into the target cell cytosol. Granzymes are part of the serine esterase family. They are closely related to other immune serine proteases expressed by innate immune cells, such as neutrophil elastase and cathepsin G.

Fas ligand is a type-II transmembrane protein expressed on various types of cells, including cytotoxic T lymphocytes, monocytes, neutrophils, breast epithelial cells, vascular endothelial cells and natural killer (NK) cells. It binds with its receptor, called FAS receptor and plays a crucial role in the regulation of the immune system and in induction of apoptosis, a programmed cell death.
The Fas receptor, also known as Fas, FasR, apoptosis antigen 1, cluster of differentiation 95 (CD95) or tumor necrosis factor receptor superfamily member 6 (TNFRSF6), is a protein that in humans is encoded by the FAS gene. Fas was first identified using a monoclonal antibody generated by immunizing mice with the FS-7 cell line. Thus, the name Fas is derived from FS-7-associated surface antigen.

The death-inducing signaling complex or DISC is a multi-protein complex formed by members of the death receptor family of apoptosis-inducing cellular receptors. A typical example is FasR, which forms the DISC upon trimerization as a result of its ligand (FasL) binding. The DISC is composed of the death receptor, FADD, and caspase 8. It transduces a downstream signal cascade resulting in apoptosis.

FAS-associated death domain protein, also called MORT1, is encoded by the FADD gene on the 11q13.3 region of chromosome 11 in humans.

The BH3 interacting-domain death agonist, or BID, gene is a pro-apoptotic member of the Bcl-2 protein family. Bcl-2 family members share one or more of the four characteristic domains of homology entitled the Bcl-2 homology (BH) domains, and can form hetero- or homodimers. Bcl-2 proteins act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities.

The p53 upregulated modulator of apoptosis (PUMA) also known as Bcl-2-binding component 3 (BBC3), is a pro-apoptotic protein, member of the Bcl-2 protein family. In humans, the Bcl-2-binding component 3 protein is encoded by the BBC3 gene. The expression of PUMA is regulated by the tumor suppressor p53. PUMA is involved in p53-dependent and -independent apoptosis induced by a variety of signals, and is regulated by transcription factors, not by post-translational modifications. After activation, PUMA interacts with antiapoptotic Bcl-2 family members, thus freeing Bax and/or Bak which are then able to signal apoptosis to the mitochondria. Following mitochondrial dysfunction, the caspase cascade is activated ultimately leading to cell death.

Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5, is a protein that, in humans, is encoded by the BIRC5 gene.

PAC-1 is a synthesized chemical compound that selectively induces apoptosis, in cancerous cells. It was granted orphan drug status by the FDA in 2016.

B-cell lymphoma-extra large (Bcl-xL), encoded by the BCL2-like 1 gene, is a transmembrane molecule in the mitochondria. It is a member of the Bcl-2 family of proteins, and acts as an anti-apoptotic protein by preventing the release of mitochondrial contents such as cytochrome c, which leads to caspase activation and ultimately, programmed cell death.

Caspase-3 is a caspase protein that interacts with caspase-8 and caspase-9. It is encoded by the CASP3 gene. CASP3 orthologs have been identified in numerous mammals for which complete genome data are available. Unique orthologs are also present in birds, lizards, lissamphibians, and teleosts.
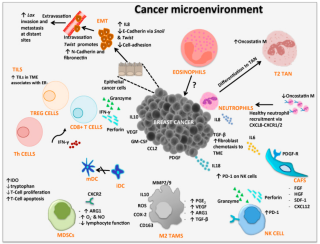
Cancer immunology (immuno-oncology) is an interdisciplinary branch of biology and a sub-discipline of immunology that is concerned with understanding the role of the immune system in the progression and development of cancer; the most well known application is cancer immunotherapy, which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.

Death receptor 4 (DR4), also known as TRAIL receptor 1 (TRAILR1) and tumor necrosis factor receptor superfamily member 10A (TNFRSF10A), is a cell surface receptor of the TNF-receptor superfamily that binds TRAIL and mediates apoptosis.

Caspase-10 is an enzyme that, in humans, is encoded by the CASP10 gene.

HU-331 is a quinone anticarcinogenic drug synthesized from cannabidiol, a cannabinoid in the Cannabis sativa plant. It showed a great efficacy against oncogenic human cells. HU-331 does not cause arrest in cell cycle, cell apoptosis or caspase activation. HU-331 inhibits DNA topoisomerase II even at nanomolar concentrations, but has shown a negligible effect on the action of DNA topoisomerase I. The cannabinoid quinone HU-331 is a very specific inhibitor of topoisomerase II, compared with most known anticancer quinones. One of the main objectives of these studies is the development of a new quinone derived compound that produces anti-neoplastic activity while maintaining low toxicity at therapeutic doses.
HAMLET is a complex between alpha-lactalbumin and oleic acid that has been shown in cell culture experiments to induce cell death in tumor cells, but not in healthy cells.

FL3 is a synthetic flavagline that displays potent anticancer and cardioprotectant activities. This compound induces the death of cancer cells by an original mechanism that involves the apoptosis-inducing factor and caspase 12, suggesting that it may improve the efficacy of cancer chemotherapies. It was also shown that FL3 may enhance the efficacy of one of the most widely used anticancer agents, doxorubicin, and alleviate its main adverse effect, cardiac damage.
Anticancer genes have a special ability to target and kill cancer cells without harming healthy ones. They do this through processes like programmed cell death, known as apoptosis, and other mechanisms like necrosis and autophagy. In the late 1990s, researchers discovered these genes while studying cancer cells. Sometimes, mutations or changes in these genes can occur, which might lead to cancer. These changes can include small alterations in the DNA sequence or larger rearrangements that affect the gene's function. When these anticancer genes are lost or altered, it can disrupt their ability to control cell growth, potentially leading to the development of cancer.
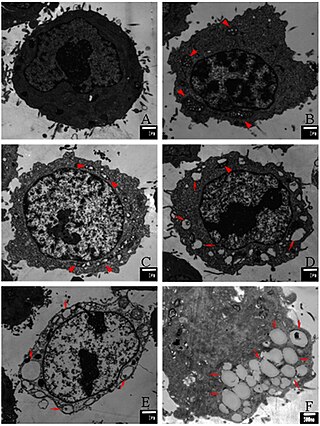
Paraptosis is a type of programmed cell death, morphologically distinct from apoptosis and necrosis. The defining features of paraptosis are cytoplasmic vacuolation, independent of caspase activation and inhibition, and lack of apoptotic morphology. Paraptosis lacks several of the hallmark characteristics of apoptosis, such as membrane blebbing, chromatin condensation, and nuclear fragmentation. Like apoptosis and other types of programmed cell death, the cell is involved in causing its own death, and gene expression is required. This is in contrast to necrosis, which is non-programmed cell death that results from injury to the cell.