The epoxyeicosatrienoic acids or EETs are signaling molecules formed within various types of cells by the metabolism of arachidonic acid by a specific subset of cytochrome P450 enzymes termed cytochrome P450 epoxygenases. These nonclassic eicosanoids are generally short-lived, being rapidly converted from epoxides to less active or inactive dihydroxy-eicosatrienoic acids (diHETrEs) by a widely distributed cellular enzyme, soluble epoxide hydrolase (sEH), also termed epoxide hydrolase 2. The EETs consequently function as transiently acting, short-range hormones; that is, they work locally to regulate the function of the cells that produce them or of nearby cells. The EETs have been most studied in animal models where they show the ability to lower blood pressure possibly by a) stimulating arterial vasorelaxation and b) inhibiting the kidney's retention of salts and water to decrease intravascular blood volume. In these models, EETs prevent arterial occlusive diseases such as heart attacks and brain strokes not only by their anti-hypertension action but possibly also by their anti-inflammatory effects on blood vessels, their inhibition of platelet activation and thereby blood clotting, and/or their promotion of pro-fibrinolytic removal of blood clots. With respect to their effects on the heart, the EETs are often termed cardio-protective. Beyond these cardiovascular actions that may prevent various cardiovascular diseases, studies have implicated the EETs in the pathological growth of certain types of cancer and in the physiological and possibly pathological perception of neuropathic pain. While studies to date imply that the EETs, EET-forming epoxygenases, and EET-inactivating sEH can be manipulated to control a wide range of human diseases, clinical studies have yet to prove this. Determination of the role of the EETS in human diseases is made particularly difficult because of the large number of EET-forming epoxygenases, large number of epoxygenase substrates other than arachidonic acid, and the large number of activities, some of which may be pathological or injurious, that the EETs possess.
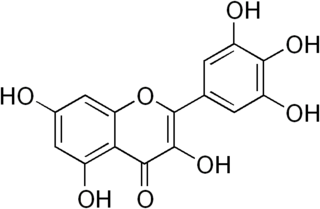
Myricetin is a member of the flavonoid class of polyphenolic compounds, with antioxidant properties. Common dietary sources include vegetables, fruits, nuts, berries, tea, and red wine.
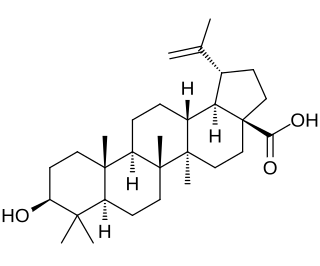
Betulinic acid is a naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase. It is found in the bark of several species of plants, principally the white birch from which it gets its name, but also the ber tree, selfheal, the tropical carnivorous plants Triphyophyllum peltatum and Ancistrocladus heyneanus, Diospyros leucomelas, a member of the persimmon family, Tetracera boiviniana, the jambul, flowering quince, rosemary, and Pulsatilla chinensis.

Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids.
The cation channels of sperm also known as Catsper channels or CatSper, are ion channels that are related to the two-pore channels and distantly related to TRP channels. The four members of this family form voltage-gated Ca2+ channels that seem to be specific to sperm. As sperm encounter the more alkaline environment of the female reproductive tract, CatSper channels become activated by the altered ion concentration. These channels are required for proper fertilization. The study of these channels has been slow because they do not traffic to the cell membrane in many heterologous systems.

Ursolic acid, is a pentacyclic triterpenoid identified in the epicuticular waxes of apples as early as 1920 and widely found in the peels of fruits, as well as in herbs and spices like rosemary and thyme.

Prolyl endopeptidase (PE) also known as prolyl oligopeptidase or post-proline cleaving enzyme is an enzyme that in humans is encoded by the PREP gene.

Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH. Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins.

Abronia villosa is a species of sand-verbena known by the common names desert sand-verbena and chaparral sand-verbena. It is in the four o'clock plant family (Nyctaginaceae). It is native to sandy areas in the deserts of the southwestern United States and northern Mexico, associated with creosote-bush and coastal-sage scrub habitats.
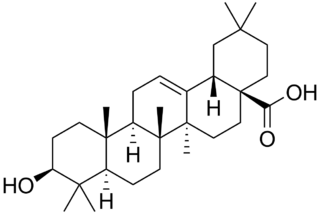
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins.

Abhydrolase domain-containing protein 2 is a serine hydrolase enzyme that is strongly expressed in human spermatozoa. It is a key controller of sperm hyperactivation, which is a necessary step in allowing sperm to fertilize an egg. It is encoded by the ABHD2 gene.

As baicalin is a flavone glycoside, it is a flavonoid. It is the glucuronide of baicalein.

An Oligopeptidase is an enzyme that cleaves peptides but not proteins. This property is due to its structure: the active site of this enzyme is located at the end of a narrow cavity which can only be reached by peptides.

A quinone methide is a type of conjugated organic compound that contain a cyclohexadiene with a carbonyl and an exocyclic methylidene or extended alkene unit. It is analogous to a quinone, but having one of the double bonded oxygens replaced with a carbon. The carbonyl and methylidene are usually oriented either ortho or para to each other. There are some examples of transient synthetic meta quinone methides.

Soluble epoxide hydrolase (sEH) is a bifunctional enzyme that in humans is encoded by the EPHX2 gene. sEH is a member of the epoxide hydrolase family. This enzyme, found in both the cytosol and peroxisomes, binds to specific epoxides and converts them to the corresponding diols. A different region of this protein also has lipid-phosphate phosphatase activity. Mutations in the EPHX2 gene have been associated with familial hypercholesterolemia.

The amyrins are three closely related natural chemical compounds of the triterpene class. They are designated α-amyrin (ursane skeleton), β-amyrin (oleanane skeleton) and δ-amyrin. Each is a pentacyclic triterpenol with the chemical formula C30H50O. They are widely distributed in nature and have been isolated from a variety of plant sources such as epicuticular wax. In plant biosynthesis, α-amyrin is the precursor of ursolic acid and β-amyrin is the precursor of oleanolic acid. All three amyrins occur in the surface wax of tomato fruit. α-Amyrin is found in dandelion coffee.

Celastrol (tripterine) is a chemical compound isolated from the root extracts of Tripterygium wilfordii and Tripterygium regelii. Celastrol is a pentacyclic nortriterpen quinone and belongs to the family of quinone methides. In mice, celastrol is an NR4A1 agonist that alleviates inflammation and induces autophagy. Also in mice, celastrol increase expression of IL1R1, which is the receptor for the cytokine interleukin-1 (IL-1). IL1R1 knock-out mice exposed to celastrol exhibit no leptin-sensitizing or anti-obesity effect.
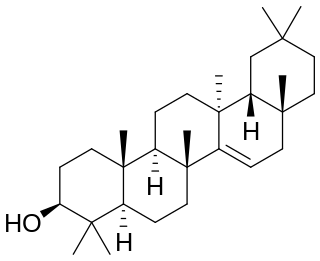
Taraxerol is a naturally-occurring pentacyclic triterpenoid. It exists in various higher plants, including Taraxacum officinale (Asteraceae), Alnus glutinosa (Betulaceae), Litsea dealbata (Lauraceae), Skimmia spp. (Rutaceae), Dorstenia spp. (Moraceae), Maytenus spp. (Celastraceae), and Alchornea latifolia (Euphobiaceae). Taraxerol was named "alnulin" when it was first isolated in 1923 from the bark of the grey alder by Zellner and Röglsperger. It also had the name "skimmiol" when Takeda and Yosiki isolated it from Skimmia (Rutaceae). A large number of medicinal plants are known to have this compound in their leaves, roots or seed oil.

Oxidosqualene cyclases (OSC) are enzymes involved in cyclization reactions of 2,3-oxidosqualene to form sterols or triterpenes.
Specialized pro-resolving mediators are a large and growing class of cell signaling molecules formed in cells by the metabolism of polyunsaturated fatty acids (PUFA) by one or a combination of lipoxygenase, cyclooxygenase, and cytochrome P450 monooxygenase enzymes. Pre-clinical studies, primarily in animal models and human tissues, implicate SPM in orchestrating the resolution of inflammation. Prominent members include the resolvins and protectins.


















