Cedrus, with the common English name cedar, is a genus of coniferous trees in the plant family Pinaceae. They are native to the mountains of the western Himalayas and the Mediterranean region, occurring at altitudes of 1,500–3,200 m (4,900–10,500 ft) in the Himalayas and 1,000–2,200 m (3,300–7,200 ft) in the Mediterranean.

Junipers are coniferous trees and shrubs in the genus Juniperus of the cypress family Cupressaceae. Depending on the taxonomy, between 50 and 67 species of junipers are widely distributed throughout the Northern Hemisphere, from the Arctic, south to tropical Africa, throughout parts of western, central and southern Asia, east to eastern Tibet in the Old World, and in the mountains of Central America. The highest-known juniper forest occurs at an altitude of 4,900 metres (16,100 ft) in southeastern Tibet and the northern Himalayas, creating one of the highest tree lines on earth.
Cypress is a common name for various coniferous trees or shrubs from the Cupressus genus of the Cupressaceae family, typically found in warm-temperate and subtropical regions of Asia, Europe, and North America.

Cupressaceae is a conifer family, the cypress, with worldwide distribution. The family includes 27–30 genera, which include the junipers and redwoods, with about 130–140 species in total. They are monoecious, subdioecious or (rarely) dioecious trees and shrubs up to 116 m (381 ft) tall. The bark of mature trees is commonly orange- to red-brown and of stringy texture, often flaking or peeling in vertical strips, but smooth, scaly or hard and square-cracked in some species.

Callitropsis nootkatensis, formerly known as Cupressus nootkatensis, is a species of tree in the cypress family native to the coastal regions of northwestern North America. This species goes by many common names including: Nootka cypress, yellow cypress, Alaska cypress, Nootka cedar, yellow cedar, Alaska cedar, and Alaska yellow cedar. The specific epithet nootkatensis is derived from the species being from the area of Nootka Sound on the west coast of Vancouver Island, Canada. Both locations are named for the older European name Nootka, given the Nuu-chah-nulth First Nation.

Thuja plicata is a large evergreen coniferous tree in the family Cupressaceae, native to the Pacific Northwest of North America. Its common name is western redcedar in the U.S. or western red cedar in the UK, and it is also called pacific red cedar, giant arborvitae, western arborvitae, just cedar, giant cedar, or shinglewood. It is not a true cedar of the genus Cedrus. T. plicata is the largest species in the genus Thuja, growing up to 70 metres (230 ft) tall and 7 m (23 ft) in diameter. It mostly grows in areas that experience a mild climate with plentiful rainfall, although it is sometimes present in drier areas on sites where water is available year-round, such as wet valley bottoms and mountain streamsides. The species is shade-tolerant and able to establish in forest understories and is thus considered a climax species. It is a very long-lived tree, with some specimens reaching ages of well over 1,000 years.

Chamaecyparis obtusa is a species of cypress native to central Japan in East Asia, and widely cultivated in the temperate northern hemisphere for its high-quality timber and ornamental qualities, with many cultivars commercially available.

The Sister Mary Grace Burns Arboretum, on the campus of Georgian Court University, in Lakewood Township, New Jersey, United States, was once the landscaped park for the winter home of George Jay Gould, millionaire son of railroad tycoon Jay Gould.

Ralph Alexander Raphael was a British organic chemist, well known for his use of acteylene derivatives in the synthesis of natural products with biological activity.

Tropolone is an organic compound with the chemical formula C7H5(OH)O. It is a pale yellow solid that is soluble in organic solvents. The compound has been of interest to research chemists because of its unusual electronic structure and its role as a ligand precursor. Although not usually prepared from tropone, it can be viewed as its derivative with a hydroxyl group in the 2-position.

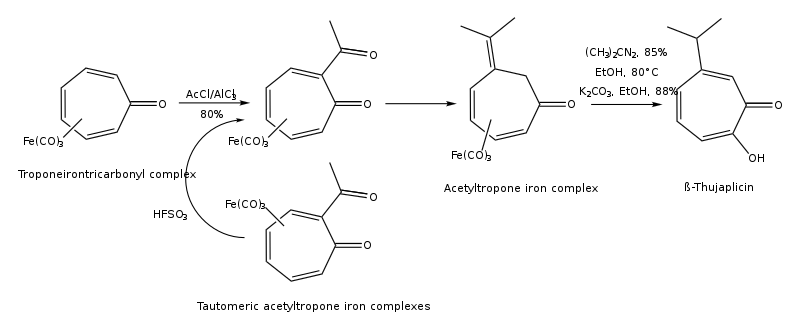
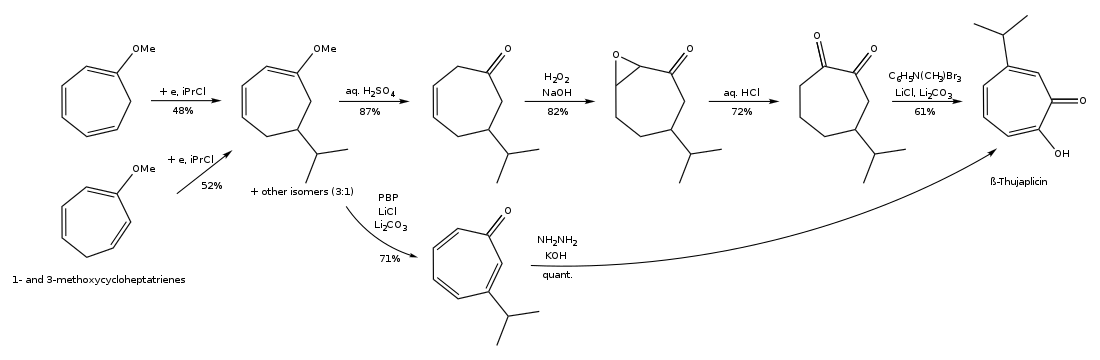
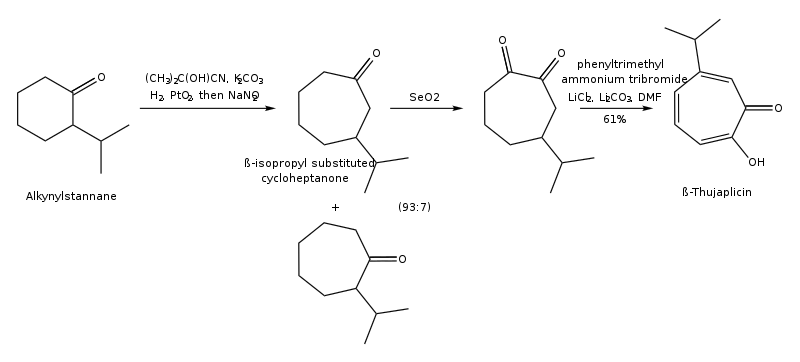
Hinokitiol (β-thujaplicin) is a natural monoterpenoid found in the wood of trees in the family Cupressaceae. It is a tropolone derivative and one of the thujaplicins. Hinokitiol is used in oral and skin care products, and is a food additive used in Japan.

Totarol is a naturally produced diterpene that is bioactive as totarol. It was first isolated by McDowell and Easterfield from the heartwood of Podocarpus totara, a conifer tree found in New Zealand. Podocarpus totara was investigated for unique molecules due to the tree's increased resistance to rotting. Recent studies have confirmed totarol's unique antimicrobial and therapeutic properties. Consequently, totarol is a candidate for a new source of drugs and has been the goal of numerous syntheses.

Cinara cupressi, the cypress aphid, is a brownish soft-bodied aphid. It sucks sap from twigs of conifers, and can cause damage to the tree, ranging from discoloring of the affected twig to the death of the tree. This insect appears to have originated in the Middle East and has been increasing its range and is considered to be an invasive species in Africa and Europe. It has been included in the List of the world's 100 worst invasive species.

Cedrol is a sesquiterpene alcohol found in the essential oil of conifers, especially in the genera Cupressus (cypress) and Juniperus (juniper). It has also been identified in Origanum onites, a plant related to oregano. Its main uses are in the chemistry of aroma compounds. It makes up about 19% of cedarwood oil Texas and 15.8% of cedarwood oil Virginia.

Mihrabat Nature Park is a nature park located on the Asian part in Beykoz district of Istanbul Province, Turkey.

Tetsuo Nozoe was a Japanese organic chemist. He is known for the discovery of hinokitiol, a seven-membered aromatic compound, and studying non-benzenoid aromatic compounds.

Thujaplicinol is either of two isomeric tropolone-related natural products. They are found in tree species primarily in bark, needles, xylem, of the family of Cupressaceae like the Cupressus, Thuja, Juniperus and Thujopsis. The thujaplicinols are structurally equivalent to the thujaplicins with an additional hydroxyl group. They belong to the class of natural terpenoids having two free hydroxyl groups at C3 and C5 position.