
Ethylenediaminetetraacetic acid (EDTA), also called EDTA acid, is an aminopolycarboxylic acid with the formula [CH2N(CH2CO2H)2]2. This white, slightly water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes even at neutral pH. It is thus used to dissolve Fe- and Ca-containing scale as well as to deliver iron ions under conditions where its oxides are insoluble. EDTA is available as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA, but these all function similarly.

Chelation therapy is a medical procedure that involves the administration of chelating agents to remove heavy metals from the body. Chelation therapy has a long history of use in clinical toxicology and remains in use for some very specific medical treatments, although it is administered under very careful medical supervision due to various inherent risks, including the mobilization of mercury and other metals through the brain and other parts of the body by the use of weak chelating agents that unbind with metals before elimination, exacerbating existing damage. To avoid mobilization, some practitioners of chelation use strong chelators, such as selenium, taken at low doses over a long period of time.

A metalloproteinase, or metalloprotease, is any protease enzyme whose catalytic mechanism involves a metal. An example is ADAM12 which plays a significant role in the fusion of muscle cells during embryo development, in a process known as myogenesis.

Dimercaprol, also called British anti-Lewisite (BAL), is a medication used to treat acute poisoning by arsenic, mercury, gold, and lead. It may also be used for antimony, thallium, or bismuth poisoning, although the evidence for those uses is not very strong. It is given by injection into a muscle.

Gadolinium(III) chloride, also known as gadolinium trichloride, is GdCl3. It is a colorless, hygroscopic, water-soluble solid. The hexahydrate GdCl3∙6H2O is commonly encountered and is sometimes also called gadolinium trichloride. Gd3+ species are of special interest because the ion has the maximum number of unpaired spins possible, at least for known elements. With seven valence electrons and seven available f-orbitals, all seven electrons are unpaired and symmetrically arranged around the metal. The high magnetism and high symmetry combine to make Gd3+ a useful component in NMR spectroscopy and MRI.

Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.
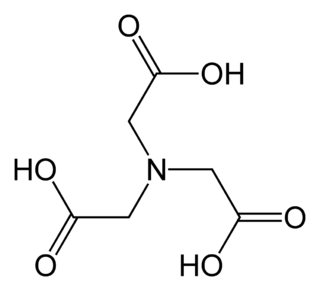
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid. Its conjugate base nitrilotriacetate is used as a chelating agent for Ca2+, Co2+, Cu2+, and Fe3+.

Pentetic acid or diethylenetriaminepentaacetic acid (DTPA) is an aminopolycarboxylic acid consisting of a diethylenetriamine backbone with five carboxymethyl groups. The molecule can be viewed as an expanded version of EDTA and is used similarly. It is a white solid with limited solubility in water.

Tetrasodium EDTA is the salt resulting from the neutralization of ethylenediaminetetraacetic acid with four equivalents of sodium hydroxide (or an equivalent sodium base). It is a white solid that is highly soluble in water. Commercial samples are often hydrated, e.g. Na4EDTA.4H2O. The properties of solutions produced from the anhydrous and hydrated forms are the same, provided they are at the same pH.

2,3-Dimercapto-1-propanesulfonic acid and its sodium salt are chelating agents that form complexes with various heavy metals. They are related to dimercaprol, which is another chelating agent.

Ethylenediamine-N,N'-disuccinic acid (EDDS) is an aminopolycarboxylic acid. It is a colourless solid that is used as chelating agent that may offer a biodegradable alternative to EDTA, which is currently used on a large scale in numerous applications.

EDDHA or ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid) is a chelating agent. Like EDTA, it binds metal ions as a hexadentate ligand, using two amines, two phenolate centers, and two carboxylates as the six binding sites. The complexes are typically anionic. The ligand itself is a white, water-soluble powder. Both the free ligand and its tetraanionic chelating agent are abbreviated EDDHA. In contrast to EDDHA, most related aminopolycarboxylic acid chelating agents feature tertiary amines and few have phenolate groups.

In coordination chemistry, denticity refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be unidentate or monodentate. Ligands with more than one bonded atom are called multidentate or polydentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms.
In coordination chemistry, a stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host–guest complexes and complexes of anions. The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine.
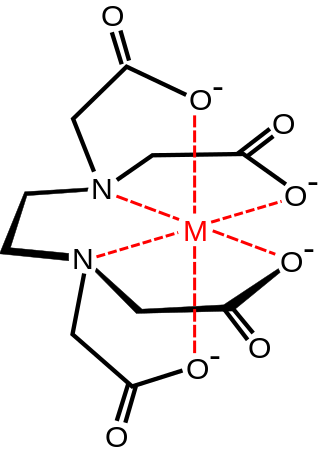
An aminopolycarboxylic acid is a chemical compound containing one or more nitrogen atoms connected through carbon atoms to two or more carboxyl groups. Aminopolycarboxylates that have lost acidic protons form strong complexes with metal ions. This property makes aminopolycarboxylic acids useful complexone in a wide variety of chemical, medical, and environmental applications.
In chemistry, binding selectivity is defined with respect to the binding of ligands to a substrate forming a complex. Binding selectivity describes how a ligand may bind more preferentially to one receptor than another. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate. Binding selectivity is of major importance in biochemistry and in chemical separation processes.

Ferric EDTA is the coordination complex formed from ferric ions and EDTA. EDTA has a high affinity for ferric ions. It gives yellowish aqueous solutions.
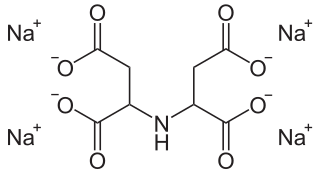
Tetrasodium iminodisuccinate is a sodium salt of iminodisuccinic acid, also referred to as N-(1,2-dicarboxyethyl)aspartic acid.
Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the transition metals. Not included in this article are complexes of the amides and ester derivatives of amino acids. Also excluded are the polyamino acids including the chelating agents EDTA and NTA.
Chelated platinum is an ionized form of platinum that forms two or more bonds with a counter ion. Some platinum chelates are claimed to have antimicrobial activity.























