
Glutathione is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine.
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease.

Schizosaccharomyces pombe, also called "fission yeast", is a species of yeast used in traditional brewing and as a model organism in molecular and cell biology. It is a unicellular eukaryote, whose cells are rod-shaped. Cells typically measure 3 to 4 micrometres in diameter and 7 to 14 micrometres in length. Its genome, which is approximately 14.1 million base pairs, is estimated to contain 4,970 protein-coding genes and at least 450 non-coding RNAs.
Cdc25 is a dual-specificity phosphatase first isolated from the yeast Schizosaccharomyces pombe as a cell cycle defective mutant. As with other cell cycle proteins or genes such as Cdc2 and Cdc4, the "cdc" in its name refers to "cell division control". Dual-specificity phosphatases are considered a sub-class of protein tyrosine phosphatases. By removing inhibitory phosphate residues from target cyclin-dependent kinases (Cdks), Cdc25 proteins control entry into and progression through various phases of the cell cycle, including mitosis and S ("Synthesis") phase.

Sulfur assimilation is the process by which living organisms incorporate sulfur into their biological molecules. In plants, sulfate is absorbed by the roots and then be transported to the chloroplasts by the transipration stream where the sulfur are reduced to sulfide with the help of a series of enzymatic reactions. Furthermore, the reduced sulfur is incorporated into cysteine, an amino acid that is a precursor to many other sulfur-containing compounds. In animals, sulfur assimilation occurs primarily through the diet, as animals cannot produce sulfur-containing compounds directly. Sulfur is incorporated into amino acids such as cysteine and methionine, which are used to build proteins and other important molecules. Besides, With the rapid development of economy, the increase emission of sulfur results in environmental issues, such as acid rain and hydrogen sulfilde.
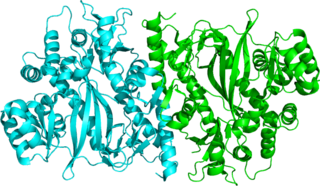
Glutathione synthetase (GSS) is the second enzyme in the glutathione (GSH) biosynthesis pathway. It catalyses the condensation of gamma-glutamylcysteine and glycine, to form glutathione. Glutathione synthetase is also a potent antioxidant. It is found in many species including bacteria, yeast, mammals, and plants.

Schizosaccharomyces is a genus of fission yeasts. The most well-studied species is S. pombe. At present five Schizosaccharomyces species have been described. Like the distantly related Saccharomyces cerevisiae, S. pombe is a significant model organism in the study of eukaryotic cell biology. It is particularly useful in evolutionary studies because it is thought to have diverged from the Saccharomyces cerevisiae lineage between 300 million and 1 billion years ago, and thus provides an evolutionarily distant comparison.

Chalcone synthase or naringenin-chalcone synthase (CHS) is an enzyme ubiquitous to higher plants and belongs to a family of polyketide synthase enzymes (PKS) known as type III PKS. Type III PKSs are associated with the production of chalcones, a class of organic compounds found mainly in plants as natural defense mechanisms and as synthetic intermediates. CHS was the first type III PKS to be discovered. It is the first committed enzyme in flavonoid biosynthesis. The enzyme catalyzes the conversion of 4-coumaroyl-CoA and malonyl-CoA to naringenin chalcone.

The acetolactate synthase (ALS) enzyme is a protein found in plants and micro-organisms. ALS catalyzes the first step in the synthesis of the branched-chain amino acids.
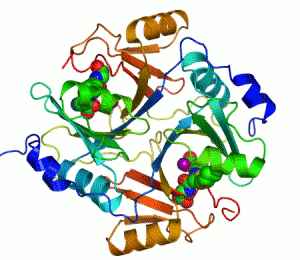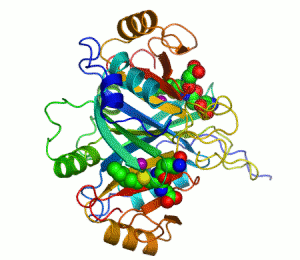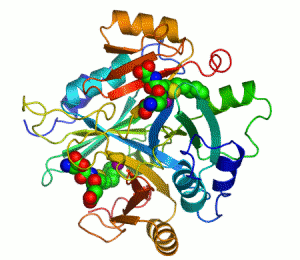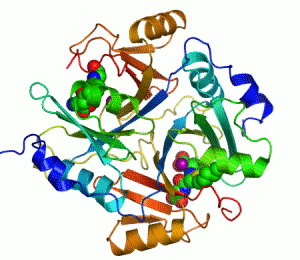
The enzyme lactoylglutathione lyase (EC 4.4.1.5, also known as glyoxalase I) catalyzes the isomerization of hemithioacetal adducts, which are formed in a spontaneous reaction between a glutathionyl group and aldehydes such as methylglyoxal.
In enzymology, a glutathione gamma-glutamylcysteinyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an initiation-specific alpha-1,6-mannosyltransferase is an enzyme that catalyzes the chemical reaction in which an alpha-D-mannosyl residue is transferred from GDP-mannose to a lipid-linked oligosaccharide, being linked by an alpha-1,6-D-mannosyl-D-mannose bond.

Structural maintenance of chromosomes protein 5 is a protein encoded by the SMC5 gene in human.

RevCen is a family of non-coding RNA found in Schizosaccharomyces. It is a megastructure containing several siRNA which use the RNAi pathway to regulate heterochromatin formation. The long RNA transcript forms a secondary structure with several stem-loops which are processed by dicer into siRNA. This siRNA then initiate the formation of heterochromatin at the centromeres of fission yeast. Northern blot analysis confirmed the siRNAs were produced from the large RNA structure RevCen in vivo. As with all siRNAs, the enzyme dicer is responsible for dissecting dsRNA into the 21nt stretch of double-stranded RNA. Human recombinant dicer enzyme processed the RevCen structure in vitro, though the same activity by yeast Dcr1 has not been confirmed.

In molecular biology, the protein domain, Ydc2, is a Holliday junction resolvase from the fission yeast Schizosaccharomyces pombe that is involved in the maintenance of mitochondrial DNA.
Throughout human history, fungi have been utilized as a source of food and harnessed to ferment and preserve foods and beverages. In the 20th century, humans have learned to harness fungi to protect human health, while industry has utilized fungi for large scale production of enzymes, acids, and biosurfactants. With the advent of modern nanotechnology in the 1980s, fungi have remained important by providing a greener alternative to chemically synthesized nanoparticle.

A septum in cell biology is the new cell wall that forms between two daughter cells as a result of cell division.

Urs Leupold was a Swiss geneticist whose studies of the fission yeast Schizosaccharomyces pombe were instrumental in establishing this organism as a key model system for eukaryotic cell and molecular biology. Leupold began his studies of S. pombe in 1946 upon encouragement by Øjvind Winge. In 1947, Leupold determined that a culture of S. pombe str. liquefaciens contained strains expressing four distinct mating types: h40, h90, h+, and h−. Most current S. pombe laboratory strains are derived from the h90, h+, and h− strains known as 968, 975, and 972.

Philip A. Rea is a British biochemist, science writer and educator, who is currently Professor of Biology and Rebecka and Arie Belldegrun Distinguished Director of the Vagelos Program in Life Sciences & Management at the University of Pennsylvania. His major contributions as a biochemist have been in the areas of membrane transport and xenobiotic detoxification, and as a science writer and educator in understanding the intersection between the life sciences and their implementation. In 2005, he and Mark V. Pauly founded the Roy and Diana Vagelos Program in Life Sciences & Management between the School of Arts and Sciences and Wharton School at the University of Pennsylvania, which he continues to co-direct in his capacity as Belldegrun Distinguished Director. Rea's work on serendipity in science has been featured in The Wall Street Journal. Additionally, he has served as a subject matter expert for 'The Scientist.
Paul Lindner was a German chemist and microbiologist, best known for discovering the fission yeast Schizosaccharomyces pombe.













