Contents
- Chemistry
- Ionization
- Optical isomerism
- History
- Synthesis
- Biosynthesis
- Industrial synthesis
- Function and uses
- Metabolism
- Neurotransmitter
- Brain nonsynaptic glutamatergic signaling circuits
- Flavor enhancer
- Nutrient
- Plant growth
- NMR spectroscopy
- Glutamate and aging
- Pharmacology
- See also
- References
- Further reading
- External links
 Skeletal formula of L-glutamic acid | |||
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name Glutamic acid | |||
| Systematic IUPAC name 2-Aminopentanedioic acid | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
| ||
| 1723801 (L) 1723799 (rac) 1723800 (D) | |||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| DrugBank | |||
| ECHA InfoCard | 100.009.567 | ||
| EC Number |
| ||
| E number | E620 (flavour enhancer) | ||
| 3502 (L) 101971 (rac) 201189 (D) | |||
| KEGG | |||
PubChem CID | |||
| UNII |
| ||
CompTox Dashboard (EPA) |
| ||
| |||
| |||
| Properties | |||
| C5H9NO4 | |||
| Molar mass | 147.130 g·mol−1 | ||
| Appearance | White crystalline powder | ||
| Density | 1.4601 (20 °C) | ||
| Melting point | 199 °C (390 °F; 472 K) decomposes | ||
| 8.57 g/L (25 °C) [1] | |||
| Solubility | Ethanol: 350 μg/100 g (25 °C) [2] | ||
| Acidity (pKa) |
| ||
| −78.5·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||
 | |||
| Warning | |||
| H315, H319, H335 | |||
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Supplementary data page | |||
| Glutamic acid (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||

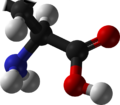
Glutamic acid (symbol Glu or E; [5] known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use.[ citation needed ] It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons.
Its molecular formula is C
5H
9NO
4. Glutamic acid exists in two optically isomeric forms; the dextrorotary L-form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation. [6] [ full citation needed ] Its molecular structure could be idealized as HOOC−CH(NH
2)−(CH
2)2−COOH, with two carboxyl groups −COOH and one amino group −NH
2. However, in the solid state and mildly acidic water solutions, the molecule assumes an electrically neutral zwitterion structure −OOC−CH(NH+
3)−(CH
2)2−COOH. It is encoded by the codons GAA or GAG.
The acid can lose one proton from its second carboxyl group to form the conjugate base, the singly-negative anion glutamate−OOC−CH(NH+
3)−(CH
2)2−COO−. This form of the compound is prevalent in neutral solutions. The glutamate neurotransmitter plays the principal role in neural activation. [7] This anion creates the savory umami flavor of foods and is found in glutamate flavorings such as monosodium glutamate (MSG). In Europe, it is classified as food additive E620. In highly alkaline solutions the doubly negative anion −OOC−CH(NH
2)−(CH
2)2−COO− prevails. The radical corresponding to glutamate is called glutamyl.
The one-letter symbol E for glutamate was assigned as the letter following D for aspartate, as glutamate is larger by one methylene –CH2– group. [8]