
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease.
Omega−3 fatty acids, also called Omega−3 oils, ω−3 fatty acids or n−3 fatty acids, are polyunsaturated fatty acids (PUFAs) characterized by the presence of a double bond, three atoms away from the terminal methyl group in their chemical structure. They are widely distributed in nature, being important constituents of animal lipid metabolism, and they play an important role in the human diet and in human physiology. The three types of omega−3 fatty acids involved in human physiology are α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). ALA can be found in plants, while DHA and EPA are found in algae and fish. Marine algae and phytoplankton are primary sources of omega−3 fatty acids. DHA and EPA accumulate in fish that eat these algae. Common sources of plant oils containing ALA include walnuts, edible seeds, and flaxseeds as well as hempseed oil, while sources of EPA and DHA include fish and fish oils, and algae oil.

Gout is a form of inflammatory arthritis characterized by recurrent attacks of a red, tender, hot and swollen joint, caused by the deposition of needle-like crystals of uric acid known as monosodium urate crystals. Pain typically comes on rapidly, reaching maximal intensity in less than 12 hours. The joint at the base of the big toe is affected (Podagra) in about half of cases. It may also result in tophi, kidney stones, or kidney damage.
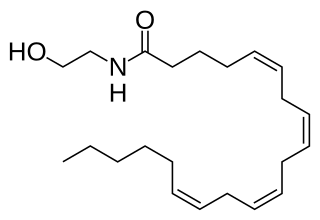
Anandamide (ANA), also known as N-arachidonoylethanolamine (AEA), an N-acylethanolamine (NAE), is a fatty acid neurotransmitter. Anandamide was the first endocannabinoid to be discovered: it participates in the body's endocannabinoid system by binding to cannabinoid receptors, the same receptors that the psychoactive compound THC in cannabis acts on. Anandamide is found in nearly all tissues in a wide range of animals. Anandamide has also been found in plants, including small amounts in chocolate. The name 'anandamide' is taken from the Sanskrit word ananda, which means "joy, bliss, delight", plus amide.

Post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes translating mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones.

Hyoscyamine is a naturally occurring tropane alkaloid and plant toxin. It is a secondary metabolite found in certain plants of the family Solanaceae, including henbane, mandrake, angel's trumpets, jimsonweed, the sorcerers' tree, and Atropa belladonna. It is the levorotary isomer of atropine and thus sometimes known as levo-atropine.

Ethanolamine is a naturally occurring organic chemical compound with the formula HOCH
2CH
2NH
2 or C
2H
7NO. The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid with an odor reminiscent of ammonia.
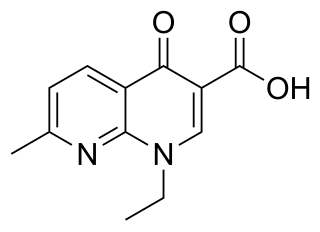
Nalidixic acid is the first of the synthetic quinolone antibiotics.

URB597 (KDS-4103) is a relatively selective and irreversible inhibitor of the enzyme fatty acid amide hydrolase (FAAH). FAAH is the primary degradatory enzyme for the endocannabinoid anandamide and, as such, inhibition of FAAH leads to an accumulation of anandamide in the CNS and periphery where it activates cannabinoid receptors. URB597 has been found to elevate anandamide levels and have activity against neuropathic pain in a mouse model.
Fatty acid desaturases are a family of enzymes that convert saturated fatty acids into unsaturated fatty acids and polyunsaturated fatty acids. For the common fatty acids of the C18 variety, desaturases convert stearic acid into oleic acid. Other desaturases convert oleic acid into linolenic acid, which is the precursor to alpha-linolenic acid, gamma-linolenic acid, and eicosatrienoic acid.

Fatty-acid amide hydrolase 1 (FAAH) is a member of the serine hydrolase family of enzymes. It was first shown to break down anandamide (AEA), an N-acylethanolamine (NAE) in 1993. In humans, it is encoded by the gene FAAH.

Phosphatidylethanolamine (PE) is a class of phospholipids found in biological membranes. They are synthesized by the addition of cytidine diphosphate-ethanolamine to diglycerides, releasing cytidine monophosphate. S-Adenosyl methionine can subsequently methylate the amine of phosphatidylethanolamines to yield phosphatidylcholines.

The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. Fatty acid metabolites with affinity for this receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA). The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1 to mediate the detection of noxious environmental stimuli.

AM404, also known as N-arachidonoylphenolamine, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid.

G protein coupled receptor 132, also termed G2A, is classified as a member of the proton sensing G protein coupled receptor (GPR) subfamily. Like other members of this subfamily, i.e. GPR4, GPR68 (OGR1), and GPR65 (TDAG8), G2A is a G protein coupled receptor that resides in the cell surface membrane, senses changes in extracellular pH, and can alter cellular function as a consequence of these changes. Subsequently, G2A was suggested to be a receptor for lysophosphatidylcholine (LPC). However, the roles of G2A as a pH-sensor or LPC receptor are disputed. Rather, current studies suggest that it is a receptor for certain metabolites of the polyunsaturated fatty acid, linoleic acid.

Choline kinase beta (CK), also known as Ethanolamine kinase (EK), Choline kinase-like protein , choline/ethanolamine kinase beta (CKEKB), or Choline/ethanolamine kinase is a protein encoded by the CHKB gene. This gene is found on chromosome 22 in humans. The encoded protein plays a key role in phospholipid biosynthesis. Choline kinase (CK) and ethanolamine kinase (EK) catalyzes the first step in phosphatidylethanolamine biosynthesis. Read-through transcripts are expressed from this locus that include exons from the downstream CPT1B locus.

An N-acylethanolamine (NAE) is a type of fatty acid amide where one of several types of acyl groups is linked to the nitrogen atom of ethanolamine, and highly metabolic formed by intake of essential fatty acids through diet by 20:4, n-6 and 22:6, n-3 fatty acids, and when the body is physically and psychologically active,. The endocannabinoid signaling system (ECS) is the major pathway by which NAEs exerts its physiological effects in animal cells with similarities in plants, and the metabolism of NAEs is an integral part of the ECS, a very ancient signaling system, being clearly present from the divergence of the protostomian/deuterostomian, and even further back in time, to the very beginning of bacteria, the oldest organisms on Earth known to express phosphatidylethanolamine, the precursor to endocannabinoids, in their cytoplasmic membranes. Fatty acid metabolites with affinity for CB receptors are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA), by brown algae which diverged about 1500 MYA, by sponges, which diverged from eumetazoans about 930 MYA, and a lineages that predate the evolution of CB receptors, as CB1 – CB2 duplication event may have occurred prior to the lophotrochozoan-deuterostome divergence 590 MYA. Fatty acid amide hydrolase (FAAH) evolved relatively recently, either after the evolution of fish 400 MYA, or after the appearance of mammals 300 MYA, but after the appearance of vertebrates. Linking FAAH, vanilloid receptors (VR1) and anandamide implies a coupling among the remaining ‘‘older’’ parts of the endocannabinoid system, monoglyceride lipase (MGL), CB receptors, that evolved prior to the metazoan–bilaterian divergence, but were secondarily lost in the Ecdysozoa, and 2-Arachidonoylglycerol (2-AG).
Palmitoylethanolamide (PEA) is an endogenous fatty acid amide, and lipid modulator PEA has been studied in in vitro and in vivo systems using exogenously added or dosed compound; there is evidence that it binds to a nuclear receptor, through which it exerts a variety of biological effects, some related to chronic inflammation and pain.
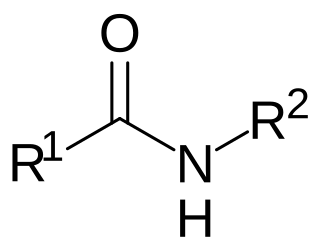
N-acyl amides are a general class of endogenous fatty acid compounds characterized by a fatty acyl group linked to a primary amine metabolite by an amide bond. Broadly speaking, N-acyl amides fall into several categories: amino acid conjugates, neurotransmitter conjugates, ethanolamine conjugates, and taurine conjugates. N-acyl amides have pleiotropic signaling functions in physiology, including in cardiovascular function, metabolic homeostasis, memory, cognition, pain, motor control and others. Initial attention focused on N-acyl amides present in mammalian organisms, however recently lipid signaling systems consisting of N-acyl amides have also been found to be present in invertebrates, such as Drosophila melanogaster. N-acyl amides play important roles in many biochemical pathways involved in a variety of physiological and pathological processes, as well as the metabolic enzymes, transporters, and receptors that regulate their signaling.
N-acylethanolamine acid amide hydrolase (NAAA) EC 3.5.1.- is a member of the choloylglycine hydrolase family, a subset of the N-terminal nucleophile hydrolase superfamily. NAAA has a molecular weight of 31 kDa. The activation and inhibition of its catalytic site is of medical interest as a potential treatment for obesity and chronic pain. While it was discovered within the last decade, its structural similarity to the more familiar acid ceramidase (AC) and functional similarity to fatty acid amide hydrolase (FAAH) allow it to be studied extensively.















