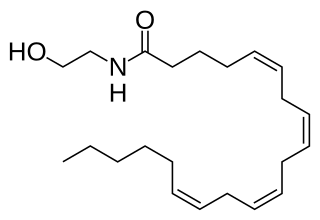
Anandamide (ANA), also referred to as N-arachidonoylethanolamine (AEA) is a fatty acid neurotransmitter belonging to the fatty acid derivative group known as N-Acylethanolamine (NAE). Anandamide takes its name from the Sanskrit word ananda, meaning "joy, bliss, delight," plus amide. Anandamide, the first discovered endocannabinoid, engages with the body's endocannabinoid system by binding to the same cannabinoid receptors that THC found in cannabis acts on. Anandamide can be found within tissues in a wide range of animals. It has also been found in plants, such as the cacao tree.

Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system of vertebrates– a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands:

Nitric oxide synthases (NOSs) are a family of enzymes catalyzing the production of nitric oxide (NO) from L-arginine. NO is an important cellular signaling molecule. It helps modulate vascular tone, insulin secretion, airway tone, and peristalsis, and is involved in angiogenesis and neural development. It may function as a retrograde neurotransmitter. Nitric oxide is mediated in mammals by the calcium-calmodulin controlled isoenzymes eNOS and nNOS. The inducible isoform, iNOS, involved in immune response, binds calmodulin at physiologically relevant concentrations, and produces NO as an immune defense mechanism, as NO is a free radical with an unpaired electron. It is the proximate cause of septic shock and may function in autoimmune disease.

Spilanthol (affinin) is a fatty acid amide isolated from Acmella oleracea. It is believed to be responsible for the local anesthetic properties of the plant.

Heřmanský–Pudlák syndrome is an extremely rare autosomal recessive disorder which results in oculocutaneous albinism, bleeding problems due to a platelet abnormality, and storage of an abnormal fat-protein compound. It is thought to affect around 1 in 500,000 people worldwide, with a significantly higher occurrence in Puerto Ricans, with a prevalence of 1 in 1800. Many of the clinical research studies on the disease have been conducted in Puerto Rico.

WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure.

The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are neurotransmitters that bind to cannabinoid receptors, and cannabinoid receptor proteins that are expressed throughout the central nervous system and peripheral nervous system. The endocannabinoid system is still not fully understood, but may be involved in regulating physiological and cognitive processes, including fertility, pregnancy, pre- and postnatal development, various activity of immune system, appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.
Nitric oxide synthase 1 (neuronal), also known as NOS1, is an enzyme that in humans is encoded by the NOS1 gene.

The cannabinoid receptor 2(CB2), is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the CNR2 gene. It is closely related to the cannabinoid receptor 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids. The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG).

Hermansky–Pudlak syndrome 4 protein is a protein that in humans is encoded by the HPS4 gene.

In medicine, exhaled nitric oxide (eNO) can be measured in a breath test for asthma and other respiratory conditions characterized by airway inflammation. Nitric oxide (NO) is a gaseous molecule produced by certain cell types in an inflammatory response. The fraction of exhaled NO (FENO) is a promising biomarker for the diagnosis, follow-up and as a guide to therapy in adults and children with asthma. The breath test has recently become available in many well-equipped hospitals in developed countries, although its exact role remains unclear.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.
Biological functions of nitric oxide are roles that nitric oxide plays within biology.

Abnormal cannabidiol (Abn-CBD) is a synthetic regioisomer of cannabidiol, which unlike most other cannabinoids produces vasodilator effects, lowers blood pressure, and induces cell migration, cell proliferation and mitogen-activated protein kinase activation in microglia, but without producing any psychoactive or sedative effects. Abn-CBD can be found as an impurity in synthetic cannabidiol.

SR144528 is a drug that acts as a potent and highly selective CB2 receptor inverse agonist, with a Ki of 0.6 nM at CB2 and 400 nM at the related CB1 receptor. It is used in scientific research for investigating the function of the CB2 receptor, as well as for studying the effects of CB1 receptors in isolation, as few CB1 agonists that do not also show significant activity as CB2 agonists are available. It has also been found to be an inhibitor of sterol O-acyltransferase, an effect that appears to be independent from its action on CB2 receptors.

S-Nitrosoglutathione (GSNO) is an endogenous S-nitrosothiol (SNO) that plays a critical role in nitric oxide (NO) signaling and is a source of bioavailable NO. NO coexists in cells with SNOs that serve as endogenous NO carriers and donors. SNOs spontaneously release NO at different rates and can be powerful terminators of free radical chain propagation reactions, by reacting directly with ROO• radicals, yielding nitro derivatives as end products. NO is generated intracellularly by the nitric oxide synthase (NOS) family of enzymes: nNOS, eNOS and iNOS while the in vivo source of many of the SNOs is unknown. In oxygenated buffers, however, formation of SNOs is due to oxidation of NO to dinitrogen trioxide (N2O3). Some evidence suggests that both exogenous NO and endogenously derived NO from nitric oxide synthases can react with glutathione to form GSNO.

AM-6545 is a drug which acts as a peripherally selective silent antagonist for the CB1 receptor, and was developed for the treatment of obesity. Other cannabinoid antagonists such as rimonabant have been marketed for this application, but have subsequently been withdrawn from sale because of centrally mediated side effects such as depression and nausea. Because AM-6545 does not cross the blood–brain barrier to any significant extent, it does not produce these kinds of side effects, but has still been shown to effectively reduce appetite and food consumption in animal studies.
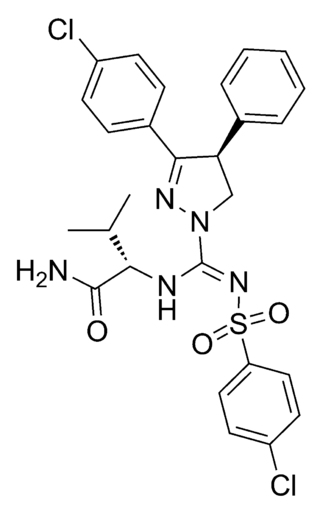
JD5037 is an antiobesity drug candidate which acts as a peripherally-restricted cannabinoid inverse agonist at CB1 receptors. It is very selective for the CB1 subtype, with a Ki of 0.35nM, >700-fold higher affinity than it has for CB2 receptors.

Monocrotaline (MCT) is a pyrrolizidine alkaloid that is present in plants of the Crotalaria genus. These species can synthesise MCT out of amino acids and can cause liver, lung and kidney damage in various organisms. Initial stress factors are released intracellular upon binding of MCT to BMPR2 receptors and elevated MAPK phosphorylation levels are induced, which can cause cancer in Homo sapiens. MCT can be detoxified in rats via oxidation, followed by glutathione-conjugation and hydrolysis.

Monlunabant is a peripherally selective cannabinoid receptor 1 inverse agonist, discovered as a β-arrestin-2-biased cannabinoid receptor 1 antagonist by Dr George Kunos, Dr Resat Cinar, and Dr Malliga Iyer at the National Institutes of Health. It was developed as a weight loss drug by Inversago Pharma.


















