Related Research Articles
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, research, and forensics. Commonly made in the laboratory by solid-phase chemical synthesis, these small fragments of nucleic acids can be manufactured as single-stranded molecules with any user-specified sequence, and so are vital for artificial gene synthesis, polymerase chain reaction (PCR), DNA sequencing, molecular cloning and as molecular probes. In nature, oligonucleotides are usually found as small RNA molecules that function in the regulation of gene expression, or are degradation intermediates derived from the breakdown of larger nucleic acid molecules.

In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.

Drug design, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that activates or inhibits the function of a biomolecule such as a protein, which in turn results in a therapeutic benefit to the patient. In the most basic sense, drug design involves the design of molecules that are complementary in shape and charge to the biomolecular target with which they interact and therefore will bind to it. Drug design frequently but not necessarily relies on computer modeling techniques. This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug design that relies on the knowledge of the three-dimensional structure of the biomolecular target is known as structure-based drug design. In addition to small molecules, biopharmaceuticals including peptides and especially therapeutic antibodies are an increasingly important class of drugs and computational methods for improving the affinity, selectivity, and stability of these protein-based therapeutics have also been developed.

High-throughput screening (HTS) is a method for scientific discovery especially used in drug discovery and relevant to the fields of biology, materials science and chemistry. Using robotics, data processing/control software, liquid handling devices, and sensitive detectors, high-throughput screening allows a researcher to quickly conduct millions of chemical, genetic, or pharmacological tests. Through this process one can quickly recognize active compounds, antibodies, or genes that modulate a particular biomolecular pathway. The results of these experiments provide starting points for drug design and for understanding the noninteraction or role of a particular location.
Functional selectivity is the ligand-dependent selectivity for certain signal transduction pathways relative to a reference ligand at the same receptor. Functional selectivity can be present when a receptor has several possible signal transduction pathways. To which degree each pathway is activated thus depends on which ligand binds to the receptor. Functional selectivity, or biased signaling, is most extensively characterized at G protein coupled receptors (GPCRs). A number of biased agonists, such as those at muscarinic M2 receptors tested as analgesics or antiproliferative drugs, or those at opioid receptors that mediate pain, show potential at various receptor families to increase beneficial properties while reducing side effects. For example, pre-clinical studies with G protein biased agonists at the μ-opioid receptor show equivalent efficacy for treating pain with reduced risk for addictive potential and respiratory depression. Studies within the chemokine receptor system also suggest that GPCR biased agonism is physiologically relevant. For example, a beta-arrestin biased agonist of the chemokine receptor CXCR3 induced greater chemotaxis of T cells relative to a G protein biased agonist.

In pharmacology, the term mechanism of action (MOA) refers to the specific biochemical interaction through which a drug substance produces its pharmacological effect. A mechanism of action usually includes mention of the specific molecular targets to which the drug binds, such as an enzyme or receptor. Receptor sites have specific affinities for drugs based on the chemical structure of the drug, as well as the specific action that occurs there.
In pharmacology, a dirty drug is an informal term for drugs that may bind to many different molecular targets or receptors in the body, and so tend to have a wide range of effects and possibly adverse drug reactions. Today, pharmaceutical companies try to make new drugs as selective as possible to minimise binding to antitargets and hence reduce the occurrence of side effects and risk of adverse reactions.

The human muscarinic acetylcholine receptor M5, encoded by the CHRM5 gene, is a member of the G protein-coupled receptor superfamily of integral membrane proteins. It is coupled to Gq protein. Binding of the endogenous ligand acetylcholine to the M5 receptor triggers a number of cellular responses such as adenylate cyclase inhibition, phosphoinositide degradation, and potassium channel modulation. Muscarinic receptors mediate many of the effects of acetylcholine in the central and peripheral nervous system. The clinical implications of this receptor have not been fully explored; however, stimulation of this receptor is known to effectively decrease cyclic AMP levels and downregulate the activity of protein kinase A (PKA).

Prostaglandin F receptor (FP) is a receptor belonging to the prostaglandin (PG) group of receptors. FP binds to and mediates the biological actions of Prostaglandin F2α (PGF2α). It is encoded in humans by the PTGFR gene.
Drug repositioning involves the investigation of existing drugs for new therapeutic purposes.
The alpha-3 beta-4 nicotinic receptor, also known as the α3β4 receptor and the ganglion-type nicotinic receptor, is a type of nicotinic acetylcholine receptor, consisting of α3 and β4 subunits. It is located in the autonomic ganglia and adrenal medulla, where activation yields post- and/or presynaptic excitation, mainly by increased Na+ and K+ permeability.

Ming-Ming Zhou is an American scientist whose specification is structural and chemical biology, NMR spectroscopy, and drug design. He is the Dr. Harold and Golden Lamport Professor and Chairman of the Department of Pharmacological Sciences. He is also the co-director of the Drug Discovery Institute at the Icahn School of Medicine at Mount Sinai and Mount Sinai Health System in New York City, as well as Professor of Sciences. Zhou is an elected fellow of the American Association for the Advancement of Science.
Phenotypic screening is a type of screening used in biological research and drug discovery to identify substances such as small molecules, peptides, or RNAi that alter the phenotype of a cell or an organism in a desired manner. Phenotypic screening must be followed up with identification and validation, often through the use of chemoproteomics, to identify the mechanisms through which a phenotypic hit works.
Chemical genetics is the investigation of the function of proteins and signal transduction pathways in cells by the screening of chemical libraries of small molecules. Chemical genetics is analogous to classical genetic screen where random mutations are introduced in organisms, the phenotype of these mutants is observed, and finally the specific gene mutation (genotype) that produced that phenotype is identified. In chemical genetics, the phenotype is disturbed not by introduction of mutations, but by exposure to small molecule tool compounds. Phenotypic screening of chemical libraries is used to identify drug targets or to validate those targets in experimental models of disease. Recent applications of this topic have been implicated in signal transduction, which may play a role in discovering new cancer treatments. Chemical genetics can serve as a unifying study between chemistry and biology. The approach was first proposed by Tim Mitchison in 1994 in an opinion piece in the journal Chemistry & Biology entitled "Towards a pharmacological genetics".

In the field of drug discovery, classical pharmacology, also known as forward pharmacology, or phenotypic drug discovery (PDD), relies on phenotypic screening of chemical libraries of synthetic small molecules, natural products or extracts to identify substances that have a desirable therapeutic effect. Using the techniques of medicinal chemistry, the potency, selectivity, and other properties of these screening hits are optimized to produce candidate drugs.
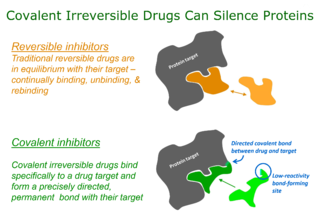
Targeted covalent inhibitors (TCIs) or Targeted covalent drugs are rationally designed inhibitors that bind and then bond to their target proteins. These inhibitors possess a bond-forming functional group of low chemical reactivity that, following binding to the target protein, is positioned to react rapidly with a proximate nucleophilic residue at the target site to form a bond.
Chemoproteomics entails a broad array of techniques used to identify and interrogate protein-small molecule interactions. Chemoproteomics complements phenotypic drug discovery, a paradigm that aims to discover lead compounds on the basis of alleviating a disease phenotype, as opposed to target-based drug discovery, in which lead compounds are designed to interact with predetermined disease-driving biological targets. As phenotypic drug discovery assays do not provide confirmation of a compound's mechanism of action, chemoproteomics provides valuable follow-up strategies to narrow down potential targets and eventually validate a molecule's mechanism of action. Chemoproteomics also attempts to address the inherent challenge of drug promiscuity in small molecule drug discovery by analyzing protein-small molecule interactions on a proteome-wide scale. A major goal of chemoproteomics is to characterize the interactome of drug candidates to gain insight into mechanisms of off-target toxicity and polypharmacology.
A proteolysis targeting chimera (PROTAC) is a heterobifunctional molecule composed of two active domains and a linker, capable of removing specific unwanted proteins. Rather than acting as a conventional enzyme inhibitor, a PROTAC works by inducing selective intracellular proteolysis. PROTACs consist of two covalently linked protein-binding molecules: one capable of engaging an E3 ubiquitin ligase, and another that binds to a target protein meant for degradation. Recruitment of the E3 ligase to the target protein results in ubiquitination and subsequent degradation of the target protein via the proteasome. Because PROTACs need only to bind their targets with high selectivity, there are currently many efforts to retool previously ineffective inhibitor molecules as PROTACs for next-generation drugs.
RNA-targeting small molecules represent a class of small molecules, organic compounds with traditional drug properties that can bind to RNA secondary or tertiary structures and alter translation patterns, localization, and degradation.
RNA therapeutics are a new class of medications based on ribonucleic acid (RNA). Research has been working on clinical use since the 1990s, with significant success in cancer therapy in the early 2010s. In 2020 and 2021, mRNA vaccines have been developed globally for use in combating the coronavirus disease. The Pfizer–BioNTech COVID-19 vaccine was the first mRNA vaccine approved by a medicines regulator, followed by the Moderna COVID-19 vaccine, and others.
References
- ↑ "Polypharmacology". PubMed. MeSH. Retrieved May 6, 2017.
- ↑ Reddy, A. Srinivas; Zhang, Shuxing (2013). "Polypharmacology: drug discovery for the future". Expert Rev Clin Pharmacol. 6 (1): 41–47. doi:10.1586/ecp.12.74. PMC 3809828 . PMID 23272792.
- ↑ Matera, Carlo; Pucci, Luca; Fiorentini, Chiara; Fucile, Sergio; Missale, Cristina; Grazioso, Giovanni; Clementi, Francesco; Zoli, Michele; De Amici, Marco; Gotti, Cecilia; Dallanoce, Clelia (2015). "Bifunctional compounds targeting both D 2 and non-α7 nACh receptors: Design, synthesis and pharmacological characterization". European Journal of Medicinal Chemistry. 101: 367–383. doi:10.1016/j.ejmech.2015.06.039. ISSN 0223-5234. PMID 26164842.
- ↑ Matera, Carlo; Bono, Federica; Pelucchi, Silvia; Collo, Ginetta; Bontempi, Leonardo; Gotti, Cecilia; Zoli, Michele; De Amici, Marco; Missale, Cristina; Fiorentini, Chiara; Dallanoce, Clelia (2019). "The novel hybrid agonist HyNDA-1 targets the D3R-nAChR heteromeric complex in dopaminergic neurons". Biochemical Pharmacology. 163: 154–168. doi:10.1016/j.bcp.2019.02.019. hdl: 2434/678632 . ISSN 0006-2952. PMID 30772268. S2CID 73466944.
- ↑ Anighoro, Andrew; Bajorath, Jürgen; Rastelli, Giulio (2014). "Polypharmacology: Challenges and Opportunities in Drug Discovery". J Med Chem. 57 (19): 7874–87. doi:10.1021/jm5006463. PMID 24946140.
- ↑ Antolin, A.A. (2014). The Impact of polypharmacology on chemical biology (Doctoral Thesis). Barcelona: Universitat Pompeu Fabra. Departament de Ciències Experimentals i de la Salut. hdl:10803/329012.
- ↑ Moellering, Raymond E.; Cravatt, Benjamin F. (January 2012). "How Chemoproteomics Can Enable Drug Discovery and Development". Chemistry & Biology. 19 (1): 11–22. doi:10.1016/j.chembiol.2012.01.001. ISSN 1074-5521. PMC 3312051 . PMID 22284350.
- ↑ Roth BL, Sheffler DJ, Kroeze WK (2004). "Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia". Nature Reviews. Drug Discovery. 3 (4): 353–9. doi:10.1038/nrd1346. PMID 15060530. S2CID 20913769.
- ↑ Gao, Huanhuan; Xiao, Jiening; Sun, Qiang; Lin, Huixian; Bai, Yunlong; Yang, Long; Yang, Baofeng; Wang, Huizhen; Wang, Zhiguo (2006-11-01). "A Single Decoy Oligodeoxynucleotides Targeting Multiple Oncoproteins Produces Strong Anticancer Effects". Molecular Pharmacology. 70 (5): 1621–1629. doi:10.1124/mol.106.024273. ISSN 0026-895X. PMID 16936227. S2CID 10019690.
- ↑ Lu, Yanjie; Xiao, Jiening; Lin, Huixian; Bai, Yunlong; Luo, Xiaobin; Wang, Zhiguo; Yang, Baofeng (Feb 2009). "A single anti-microRNA antisense oligodeoxyribonucleotide (AMO) targeting multiple microRNAs offers an improved approach for microRNA interference". Nucleic Acids Research. 37 (3): e24. doi:10.1093/nar/gkn1053. ISSN 1362-4962. PMC 2647303 . PMID 19136465.
- ↑ Wang, Zhiguo (2009). MicroRNA Interference Technologies. doi:10.1007/978-3-642-00489-6. ISBN 978-3-642-00488-9.
- ↑ Wang, Zhiguo (2011). "The concept of multiple-target anti-miRNA antisense oligonucleotide technology". MicroRNA and Cancer. Methods in Molecular Biology. Vol. 676. pp. 51–57. doi:10.1007/978-1-60761-863-8_4. ISBN 978-1-60761-862-1. ISSN 1940-6029. PMID 20931389.
- ↑ Wang, Zhiguo; Yang, Baofeng (2022). "Polypharmacology: Principles and Methodologies". Springer Nature (Switzerland AG). ISBN 978-3-031-04998-9.
- ↑ Wang, Zhiguo (2024). "Anti-Aging Polypharmacology". Cambridge Scholars Publishing (UK). ISBN 978-1-0364-0680-6.
- ↑ Tomaselli, D.; Lucidi, A.; Rotili, D.; Mai, A. (2020). "Epigenetic polypharmacology: A new frontier for epi-drug discovery. | wizdom.ai - intelligence for everyone". Medicinal Research Reviews. 40 (1): 190–244. doi:10.1002/MED.21600. PMC 6917854 . PMID 31218726.
- ↑ Apsel, Beth; Blair, Jimmy A.; Gonzalez, Beatriz; Nazif, Tamim M.; Feldman, Morri E.; Aizenstein, Brian; Hoffman, Randy; Williams, Roger L.; Shokat, Kevan M.; Knight, Zachary A. (Oct 2008). "Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases". Nature Chemical Biology. 4 (11): 691–699. doi:10.1038/nchembio.117. ISSN 1552-4469. PMC 2880455 . PMID 18849971.
- ↑ Reddy, A Srinivas; Zhang, Shuxing (2013-01-01). "Polypharmacology: drug discovery for the future". Expert Review of Clinical Pharmacology. 6 (1): 41–47. doi:10.1586/ecp.12.74. ISSN 1751-2433. PMC 3809828 . PMID 23272792.