
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system of vertebrates– a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands:
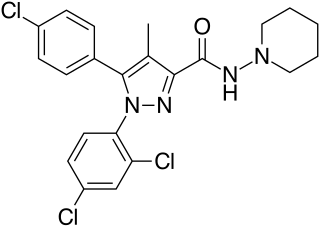
Rimonabant (also known as SR141716; trade names Acomplia, Zimulti) is an anorectic antiobesity drug approved in Europe in 2006 but was withdrawn worldwide in 2008 due to serious psychiatric side effects; it was never approved in the United States. Rimonabant is an inverse agonist for the cannabinoid receptor CB1 and was first-in-class for clinical development.

The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are neurotransmitters that bind to cannabinoid receptors, and cannabinoid receptor proteins that are expressed throughout the central nervous system and peripheral nervous system. The endocannabinoid system is still not fully understood, but may be involved in regulating physiological and cognitive processes, including fertility, pregnancy, pre- and postnatal development, various activity of immune system, appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.

AM404, also known as N-arachidonoylphenolamine, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid. AM404 is one of the AM cannabinoids discovered by Alexandros Makriyannis and his team.

Cannabinoid receptor 1 (CB1), is a G protein-coupled cannabinoid receptor that in humans is encoded by the CNR1 gene. And discovered, by determination and characterization in 1988, and cloned in 1990 for the first time. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by endogenous cannabinoids called endocannabinoids, a group of retrograde neurotransmitters that include lipids, such as anandamide and 2-arachidonoylglycerol; plant phytocannabinoids, such as docosatetraenoylethanolamide found in wild daga, the compound tetrahydrocannabinol which is an active constituent of the psychoactive drug cannabis; and synthetic analogs of tetrahydrocannabinol. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin at low doses and at higher doses, it activate the CB1 receptor as an agonist, but with less potency than tetrahydrocannabinol.

The cannabinoid receptor 2(CB2), is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the CNR2 gene. It is closely related to the cannabinoid receptor 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids. The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG).

NESS-0327 is a drug used in scientific research which acts as an extremely potent and selective antagonist of the cannabinoid receptor CB1. It is much more potent an antagonist, and more selective for the CB1 receptor over CB2, than the more commonly used ligand rimonabant, with a Ki at CB1 of 350fM (i.e. 0.00035nM) and a selectivity of over 60,000x for CB1 over CB2. Independently, two other groups have described only modest nanomolar CB1 affinity for this compound (125nM and 18.4nM). Also unlike rimonabant, NESS-0327 does not appear to act as an inverse agonist at higher doses, instead being a purely neutral antagonist which blocks the CB1 receptor but does not produce any physiological effect of its own.

Surinabant (SR147778) is a cannabinoid receptor type 1 antagonist developed by Sanofi-Aventis. It is being investigated as a potential treatment for nicotine addiction, to assist smoking cessation. It may also be developed as an anorectic drug to assist with weight loss, however there are already several CB1 antagonists or inverse agonists on the market or under development for this application, so surinabant is at present mainly being developed as an anti-smoking drug, with possible application in the treatment of other addictive disorders such as alcoholism. Other potential applications such as treatment of ADHD have also been proposed.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.
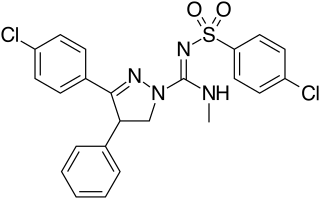
Ibipinabant (SLV319, BMS-646,256) is a drug used in scientific research which acts as a potent and highly selective CB1 antagonist. It has potent anorectic effects in animals, and was researched for the treatment of obesity, although CB1 antagonists as a class have now fallen out of favour as potential anorectics following the problems seen with rimonabant, and so ibipinabant is now only used for laboratory research, especially structure-activity relationship studies into novel CB1 antagonists. SLV330, which is a structural analogue of Ibipinabant, was reported active in animal models related to the regulation of memory, cognition, as well as in addictive behavior. An atom-efficient synthesis of ibipinabant has been reported.

AM-1241 (1-(methylpiperidin-2-ylmethyl)-3-(2-iodo-5-nitrobenzoyl)indole) is a chemical from the aminoalkylindole family that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 3.4 nM at CB2 and 80 times selectivity over the related CB1 receptor. It has analgesic effects in animal studies, particularly against "atypical" pain such as hyperalgesia and allodynia. This is thought to be mediated through CB2-mediated peripheral release of endogenous opioid peptides, as well as direct activation of the TRPA1 channel. It has also shown efficacy in the treatment of amyotrophic lateral sclerosis in animal models.
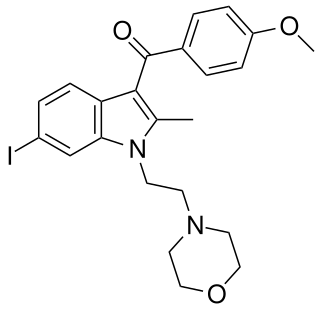
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.

TM-38837 is a small molecule inverse agonist/antagonist of the CB1 cannabinoid receptor, with peripheral selectivity. It is being developed for the treatment of obesity and metabolic disorders by 7TM Pharma. The company has announced phase I clinical trials.

SR144528 is a drug that acts as a potent and highly selective CB2 receptor inverse agonist, with a Ki of 0.6 nM at CB2 and 400 nM at the related CB1 receptor. It is used in scientific research for investigating the function of the CB2 receptor, as well as for studying the effects of CB1 receptors in isolation, as few CB1 agonists that do not also show significant activity as CB2 agonists are available. It has also been found to be an inhibitor of sterol O-acyltransferase, an effect that appears to be independent from its action on CB2 receptors.

O-1269 is a drug that is a diarylpyrazole derivative, related to potent cannabinoid antagonist drugs such as rimonabant and surinabant. However O-1269 and several related drugs were unexpectedly found to act as full or partial agonists at the cannabinoid receptors rather than antagonists, and so produce the usual effects expected of cannabinoid agonists in animal tests, such as sedation and analgesic effects. The N-heptyl homolog O-1270 and the N-propyl homolog O-1399 also act as cannabinoid agonists with similar potency in vivo, despite weaker binding affinity at cannabinoid receptors compared to the pentyl homolog O-1269. Agonist-like and atypical cannabinoid activity has also been observed with a number of related compounds.
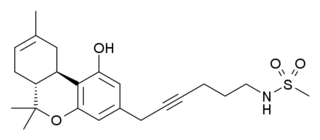
O-2050 is a drug that is a classical cannabinoid derivative, which acts as an antagonist for the CB1 receptor. This gives it an advantage in research over many commonly used cannabinoid antagonists, such as rimonabant, which at higher doses act as inverse agonists at CB1 as well as showing off-target effects. However, while O-2050 acts as a silent antagonist in vitro, some tests in vivo have suggested it may show agonist activity under certain circumstances.

Rosonabant (INN; E-6776) is a drug acting as a CB1 receptor antagonist/inverse agonist that was under investigation by Esteve as an appetite suppressant for the treatment of obesity. Development of the drug for clinical use was apparently halted shortly after the related CB1 antagonist rimonabant was discontinued in November 2008, due to the reports of severe psychiatric adverse effects such as anxiety, depression, and suicidal ideation associated with it and with similarly acting agents.
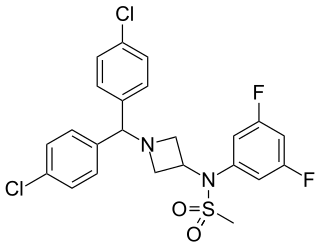
Drinabant (INN; AVE-1625) is a drug that acts as a selective CB1 receptor antagonist, which was under investigation varyingly by Sanofi-Aventis as a treatment for obesity, schizophrenia, Alzheimer's disease, Parkinson's disease, and nicotine dependence. Though initially studied as a potential treatment for a variety of different medical conditions, Sanofi-Aventis eventually narrowed down the therapeutic indications of the compound to just appetite suppression. Drinabant reached phase IIb clinical trials for this purpose in the treatment of obesity but was shortly thereafter discontinued, likely due to the observation of severe psychiatric side effects including anxiety, depression, and thoughts of suicide in patients treated with the now-withdrawn rimonabant, another CB1 antagonist that was also under development by Sanofi-Aventis.
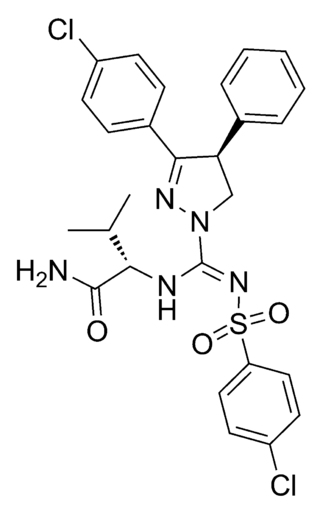
JD5037 is an antiobesity drug candidate which acts as a peripherally-restricted cannabinoid inverse agonist at CB1 receptors. It is very selective for the CB1 subtype, with a Ki of 0.35nM, >700-fold higher affinity than it has for CB2 receptors.

















