
MN 18 is an indazole-based synthetic cannabinoid that is an agonist for the cannabinoid receptors, with Ki values of 45.72 nM at CB1 and 11.098 nM at CB2 and EC50 values of 2.028 nM at CB1 and 1.233 nM at CB2, and has been sold online as a designer drug. It is the indazole core analogue of NNE1. Given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, it is suspected that metabolic hydrolysis of the amide group of MN-18 may release 1-naphthylamine, a known carcinogen. MN-18 metabolism has been described in literature.

AM-2201 is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor. It is part of the AM series of cannabinoids discovered by Alexandros Makriyannis at Northeastern University.

APINACA (AKB48, N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide) is a drug that acts as a reasonably potent agonist for the cannabinoid receptors. It is a full agonist at CB1 with an EC50 of 142 nM and Ki of 3.24 nM (compared to the Ki of Δ9-THC at 28.35 nM and JWH-018 at 9.62 nM), while at CB2 it acts as a partial agonist with an EC50 of 141 nM and Ki of 1.68 nM (compared to the Ki of Δ9-THC at 37.82 nM and JWH-018 at 8.55 nM). Its pharmacological characterization has also been reported in a discontinued patent application. It had never previously been reported in the scientific or patent literature, and was first identified by laboratories in Japan in March 2012 as an ingredient in synthetic cannabis smoking blends, along with a related compound APICA. Structurally, it closely resembles cannabinoid compounds from a University of Connecticut patent, but with a simple pentyl chain on the indazole 1-position, and APINACA falls within the claims of this patent despite not being disclosed as an example.

5F-PB-22 is a designer drug which acts as a cannabinoid agonist. The structure of 5F-PB-22 appears to have been designed with an understanding of structure–activity relationships within the indole class of cannabinoids.

SDB-006 is a drug that acts as a potent agonist for the cannabinoid receptors, with an EC50 of 19 nM for human CB2 receptors, and 134 nM for human CB1 receptors. It was discovered during research into the related compound SDB-001 which had been sold illicitly as "2NE1". SDB-006 metabolism has been described in literature.
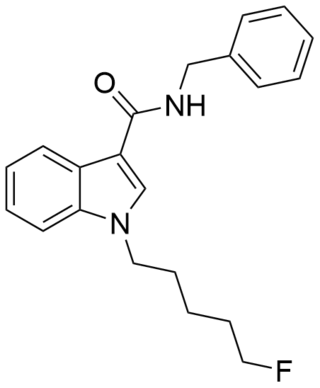
5F-SDB-006 is a drug that acts as a potent agonist for the cannabinoid receptors, with an EC50 of 50 nM for human CB1 receptors, and 123 nM for human CB2 receptors. It was discovered during research into the related compound APICA which had been sold illicitly as "2NE1". 5F-SDB-006 is the terminally fluorinated analog of SDB-006, just as STS-135 is the terminally fluorinated analog of APICA. Given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, it is suspected that metabolic hydrolysis of the amide group of 5F-SDB-006 may release benzylamine.

SDB-005 is an indazole-based synthetic cannabinoid that has been sold online as a designer drug. It is presumed to be an agonist of the CB1 and CB2 cannabinoid receptors. SDB-005 is the indazole core analog of PB-22 where the 8-hydroxyquinoline has also been replaced with a naphthalene group.
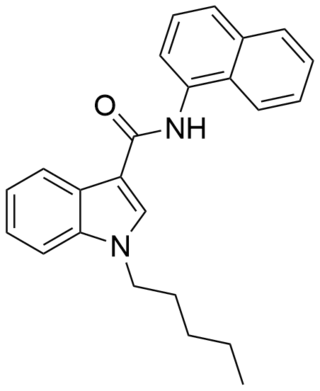
NNE1 (also known as NNEI, MN-24 and AM-6527) is an indole-based synthetic cannabinoid, representing a molecular hybrid of APICA and JWH-018 that is an agonist for the cannabinoid receptors, with Ki values of 60.09 nM at CB1 and 45.298 nM at CB2 and EC50 values of 9.481 nM at CB1 and 1.008 nM at CB2. It was invented by Abbott and has a CB1 receptor pEC50 of 8.9 with around 80x selectivity over the related CB2 receptor. It is suspected that metabolic hydrolysis of the amide group of NNE1 may release 1-naphthylamine, a known carcinogen, given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, and NNE1 was banned in New Zealand in 2012 as a temporary class drug to stop it being used as an ingredient in then-legal synthetic cannabis products. NNE1 was subsequently found to be responsible for the death of a man in Japan in 2014.

CUMYL-PICA (SGT-56) is an indole-3-carboxamide based synthetic cannabinoid. It is the α,α-dimethylbenzyl analogue of SDB-006. It was briefly sold in New Zealand during 2013 as an ingredient of at the time legal synthetic cannabis products, but the product containing CUMYL-BICA and CUMYL-PICA was denied an interim licensing approval under the Psychoactive Substances regulatory scheme, due to reports of adverse events in consumers. CUMYL-PICA acts as an agonist for the cannabinoid receptors, with Ki values of 59.21 nM at CB1 and 136.38 nM at CB2 and EC50 values of 11.98 nM at CB1 and 16.2 nM at CB2.

NM-2201 (also known as CBL-2201 and NA-5F-PIC) is an indole-based synthetic cannabinoid that presumably has similar properties to the closely related 5F-PB-22 and NNE1, which are both full agonists and unselectively bind to CB1 and CB2 receptors with low nanomolar affinity.

5F-CUMYL-PINACA (also known as SGT-25 and sometimes sold in e-cigarette form as C-Liquid) is an indazole-3-carboxamide based synthetic cannabinoid. 5F-CUMYL-PINACA acts as a potent agonist for the cannabinoid receptors, with the original patent claiming approximately 4x selectivity for CB1, having an EC50 of <0.1 nM for human CB1 receptors and 0.37 nM for human CB2 receptors. In more recent assays using different techniques, 5F-CUMYL-PINACA was variously found to have an EC50 of 0.43 nM at CB1 and 11.3 nM at CB2, suggesting a somewhat higher CB1 selectivity of 26 times, or alternatively 15.1 nM at CB1 and 34.8 nM at CB2 with only 2.3 times selectivity, however these figures cannot be directly compared due to the different assay techniques used in each case.

CUMYL-THPINACA (also known as SGT-42) is an indazole-3-carboxamide based synthetic cannabinoid. CUMYL-THPINACA acts as a potent agonist for the cannabinoid receptors, with approximately 6x selectivity for CB1, having an EC50 of 0.1nM for human CB1 receptors and 0.59nM for human CB2 receptors.

CUMYL-PINACA (also known as SGT-24) is an indazole-3-carboxamide based synthetic cannabinoid. CUMYL-PINACA acts as a potent agonist for the cannabinoid receptors, with approximately 3x selectivity for CB1, having an EC50 of 0.15nM for human CB1 receptors and 0.41nM for human CB2 receptors. In its pure form, it is described as a sticky oil which can cause poisoning through transdermal exposure.
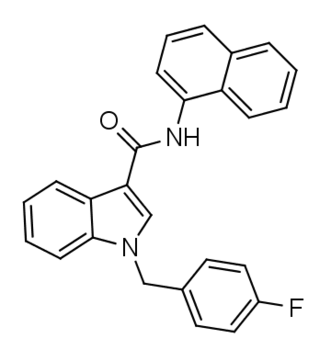
FDU-NNE1 (also known as FDU-NNEI and FDU-MN-24) is an indole-based synthetic cannabinoid that is presumed to be a potent agonist of the CB1 receptor and has been sold online as a designer drug. Given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, it is suspected that metabolic hydrolysis of the amide group of FDU-NNE1 will release 1-naphthylamine, a known carcinogen.
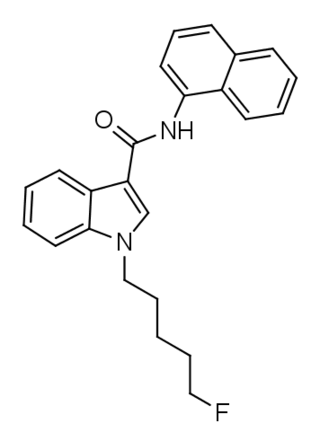
5F-NNE1 (also known as 5F-NNEI and 5F-MN-24) is an indole-based synthetic cannabinoid that is presumed to be a potent agonist of the CB1 receptor and has been sold online as a designer drug. Given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, it is suspected that metabolic hydrolysis of the amide group of 5F-NNE1 may release 1-naphthylamine, a known carcinogen.
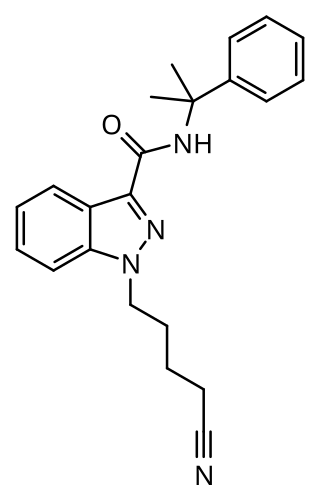
CUMYL-4CN-BINACA (also known as CUMYL-CYBINACA or SGT-78) is an indazole-3-carboxamide based synthetic cannabinoid that has been sold online as a designer drug. It is a potent agonist for cannabinoid receptors CB1 and CB2, with in vitro EC50 values of 0.58 nM and 6.12 nM, respectively. In mice, CUMYL-4CN-BINACA produces hypothermic and pro-convulsant effects via the CB1 receptor, and anecdotal reports suggest it has an active dose of around 0.1 mg in humans.

5F-CUMYL-P7AICA is a pyrrolo[2,3-b]pyridine-3-carboxamide based synthetic cannabinoid that has been sold as a designer drug. It was first identified by the EMCDDA in February 2015.

5F-CUMYL-PEGACLONE (5F-SGT-151, SGT-269) is a gamma-carboline based synthetic cannabinoid that has been sold as a designer drug, first being identified in Germany in 2017. It acts as a potent full agonist of the CB1 receptor. It appears to be more toxic than related compounds such as CUMYL-PEGACLONE, and has been linked to numerous serious adverse reactions, some fatal.

CUMYL-CBMICA (SGT-280) is an indole-3-carboxamide based synthetic cannabinoid receptor agonist which has been sold as a designer drug, first being identified in Germany in August 2019. Since the structure fell outside the German drug analogue law provisions at the time, an amendment was made to the law to expand the relevant definition, which came into effect in April 2020. It has been shown to act as a CB1 receptor agonist with an EC50 of 62.9nM.



















