
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradation occurs under a specific set of circumstances.
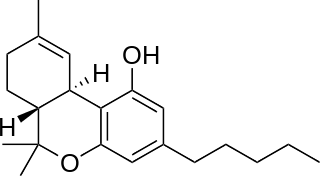
Tetrahydrocannabinol (THC) is a cannabinoid found in cannabis. It is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term THC usually refers to the delta-9-THC isomer with chemical name (−)-trans-Δ9-tetrahydrocannabinol. It is a colorless oil.

A cannabis edible, also known as a cannabis-infused food or simply an edible, is a food item that contains decarboxylated cannabinoids from cannabis extract as an active ingredient. Although edible may refer to either a food or a drink, a cannabis-infused drink may be referred to more specifically as a liquid edible or drinkable. Edibles are a way to consume cannabis. Unlike smoking, in which cannabinoids are inhaled into the lungs and pass rapidly into the bloodstream, peaking in about ten minutes and wearing off in a couple of hours, cannabis edibles may take hours to digest, and their effects may peak two to three hours after consumption and persist for around six hours. The food or drink used may affect both the timing and potency of the dose ingested.

Medical cannabis, medicinal cannabis or medical marijuana (MMJ) refers to cannabis products and cannabinoid molecules that are prescribed by physicians for their patients. The use of cannabis as medicine has a long history, but has not been as rigorously tested as other medicinal plants due to legal and governmental restrictions, resulting in limited clinical research to define the safety and efficacy of using cannabis to treat diseases.

Hemp, or industrial hemp, is a plant in the botanical class of Cannabis sativa cultivars grown specifically for industrial and consumable use. It can be used to make a wide range of products. Along with bamboo, hemp is among the fastest growing plants on Earth. It was also one of the first plants to be spun into usable fiber 50,000 years ago. It can be refined into a variety of commercial items, including paper, rope, textiles, clothing, biodegradable plastics, paint, insulation, biofuel, food, and animal feed.

Cannabis sativa is an annual herbaceous flowering plant. The species was first classified by Carl Linnaeus in 1753. The specific epithet sativa means 'cultivated'. Indigenous to Eastern Asia, the plant is now of cosmopolitan distribution due to widespread cultivation. It has been cultivated throughout recorded history and used as a source of industrial fiber, seed oil, food, and medicine. It is also used as a recreational drug and for religious and spiritual purposes.

Cannabidiol (CBD) is a phytocannabinoid, one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. Medically, it is an anticonvulsant used to treat multiple forms of epilepsy. It was discovered in 1940 and, as of 2024 clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient high-quality evidence that CBD is effective for these conditions. CBD is sold as an herbal dietary supplement and promoted with yet unproven claims of particular therapeutic effects.

Linalool refers to two enantiomers of a naturally occurring terpene alcohol found in many flowers and spice plants. Together with geraniol, nerol, citronellol, linalool is one of the rose alcohols. Linalool has multiple commercial applications, the majority of which are based on its pleasant scent.

Cannabis, commonly known as marijuana, weed, and pot, among other names, is a non-chemically uniform drug from the cannabis plant. Native to Central or South Asia, the cannabis plant has been used as a drug for both recreational and entheogenic purposes and in various traditional medicines for centuries. Tetrahydrocannabinol (THC) is the main psychoactive component of cannabis, which is one of the 483 known compounds in the plant, including at least 65 other cannabinoids, such as cannabidiol (CBD). Cannabis can be used by smoking, vaporizing, within food, or as an extract.

Eucalyptol is a monoterpenoid colorless liquid, and a bicyclic ether. It has a fresh camphor-like odor and a spicy, cooling taste. It is insoluble in water, but miscible with organic solvents. Eucalyptol makes up about 70–90% of eucalyptus oil. Eucalyptol forms crystalline adducts with hydrohalic acids, o-cresol, resorcinol, and phosphoric acid. Formation of these adducts is useful for purification.

Myrcene, or β-myrcene, is a monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from Myrcia, from which it gets its name. It is an intermediate in the production of several fragrances. An less-common isomeric form, having one of the three alkene units in a different position, is α-myrcene.

Cannabigerol (CBG) is a non-psychoactive cannabinoid and minor constituent of cannabis. It is one of more than 120 identified cannabinoids found in the plant genus Cannabis. The compound is the decarboxylated form of cannabigerolic acid (CBGA), the parent molecule from which other cannabinoids are biosynthesized.

Cbl is a mammalian gene family. CBL gene, a part of the Cbl family, encodes the protein CBL which is an E3 ubiquitin-protein ligase involved in cell signalling and protein ubiquitination. Mutations to this gene have been implicated in a number of human cancers, particularly acute myeloid leukaemia.

Cannabichromene (CBC), also called cannabichrome, cannanbichromene, pentylcannabichromene or cannabinochromene, exhibits anti-inflammatory properties in vitro, which may, theoretically, contribute to cannabis analgesic effects. It is a phytocannabinoid, one of the hundreds of cannabinoids found in the Cannabis plant. It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol (THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabinol (CBN), among others. CBC and cannabinols are present in cannabis. It is not scheduled by the Convention on Psychotropic Substances.
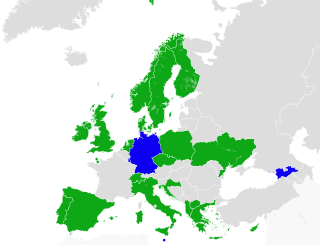
Cannabis in France is illegal for personal use, but remains one of the most popular illegal drugs. Limited types of cannabis-derived products are permitted for medical uses.
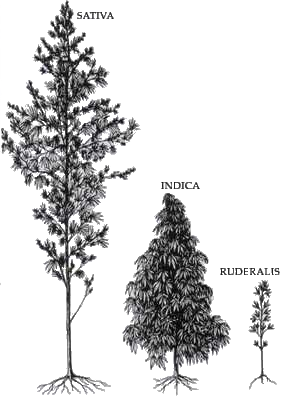
The following outline is provided as an overview of and topical guide to the plant Cannabis sativa and its relatives Cannabis indica and Cannabis ruderalis, the drug cannabis (drug) and the industrial product hemp.

Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 receptor agonist which was known as a synthetic homologue of tetrahydrocannabinol (THC), but for the first time in 2019 was isolated as a natural product in trace amounts from Cannabis sativa.

Hexahydrocannabinol (HHC) is a hydrogenated derivative of tetrahydrocannabinol (THC). It is a naturally occurring phytocannabinoid that has rarely been identified as a trace component in Cannabis sativa, but can also be produced synthetically by firstly acid cyclization of cannabidiol and then hydrogenation of tetrahydrocannabinol. The synthesis and bioactivity of HHC was first reported in 1940 by Roger Adams.

Cannabicitran (CBTC) is a phytocannabinoid first isolated in 1974 as a trace component of Cannabis sativa. Structurally related compounds can be found in some other plants. It is not psychoactive, but was found to reduce intraocular pressure in tests on rabbits, which may reflect agonist activity at the NAGly receptor that is known to be a target of many structurally related cannabinoids.

Cannabimovone (CBM) is a phytocannabinoid first isolated from a non-psychoactive strain of Cannabis sativa in 2010, which is thought to be a rearrangement product of cannabidiol. It lacks affinity for cannabinoid receptors, but acts as an agonist at both TRPV1 and PPARγ.




















