
Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 100 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Helenalin, or (-)-4-Hydroxy-4a,8-dimethyl-3,3a,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione, is a toxic sesquiterpene lactone which can be found in several plants such as Arnica montana and Arnica chamissonis Helenalin is responsible for the toxicity of the Arnica spp. Although toxic, helenalin possesses some in vitro anti-inflammatory and anti-neoplastic effects. Helenalin can inhibit certain enzymes, such as 5-lipoxygenase and leukotriene C4 synthase. For this reason the compound or its derivatives may have potential medical applications.

The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.
Arachidonate 5-lipoxygenase, also known as ALOX5, 5-lipoxygenase, 5-LOX, or 5-LO, is a non-heme iron-containing enzyme that in humans is encoded by the ALOX5 gene. Arachidonate 5-lipoxygenase is a member of the lipoxygenase family of enzymes. It transforms essential fatty acids (EFA) substrates into leukotrienes as well as a wide range of other biologically active products. ALOX5 is a current target for pharmaceutical intervention in a number of diseases.
Arachidonate 5-lipoxygenase inhibitors are compounds that slow or stop the action of the arachidonate 5-lipoxygenase enzyme, which is responsible for the production of inflammatory leukotrienes. The overproduction of leukotrienes is a major cause of inflammation in asthma, allergic rhinitis, and osteoarthritis.

1-Phenyl-2-propylaminopentane is an experimental drug related to selegiline which acts as a catecholaminergic activity enhancer (CAE).

ALOX15 is, like other lipoxygenases, a seminal enzyme in the metabolism of polyunsaturated fatty acids to a wide range of physiologically and pathologically important products. ▼ Gene Function

ALOX12, also known as arachidonate 12-lipoxygenase, 12-lipoxygenase, 12S-Lipoxygenase, 12-LOX, and 12S-LOX is a lipoxygenase-type enzyme that in humans is encoded by the ALOX12 gene which is located along with other lipoyxgenases on chromosome 17p13.3. ALOX12 is 75 kilodalton protein composed of 663 amino acids.

G protein-coupled receptor 55 also known as GPR55 is a G protein-coupled receptor that in humans is encoded by the GPR55 gene.

Cysteinyl leukotriene receptor 2, also termed CYSLTR2, is a receptor for cysteinyl leukotrienes (LT). CYSLTR2, by binding these cysteinyl LTs contributes to mediating various allergic and hypersensitivity reactions in humans. However, the first discovered receptor for these CsLTs, cysteinyl leukotriene receptor 1 (CysLTR1), appears to play the major role in mediating these reactions.
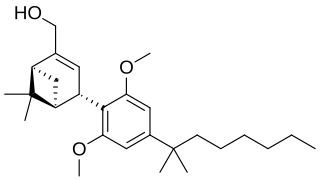
Onternabez (also known as HU-308, HU308, PPP-003, and ARDS-003) is a synthetic cannabinoid that acts as a potent cannabinoid agonist. It is highly selective for the cannabinoid-2 receptor (CB2 receptor) subtype, with a selectivity more than 5,000 times greater for the CB2 receptor than the CB1 receptor. The synthesis and characterization of onternabez took place in the laboratory of Raphael Mechoulam at the Hebrew University of Jerusalem (the HU in HU-308) in the late 1990s. The pinene dimethoxy-DMH-CBD derivative onternabez was identified as a potent peripheral CB2-selective agonist in in vitro and animal studies in 1990 and 1999.
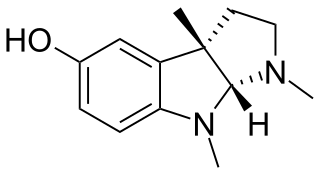
Eseroline is a drug which acts as an opioid agonist. It is a metabolite of the acetylcholinesterase inhibitor physostigmine but unlike physostigmine, the acetylcholinesterase inhibition produced by eseroline is weak and easily reversible, and it produces fairly potent analgesic effects mediated through the μ-opioid receptor. This mixture of activities gives eseroline an unusual pharmacological profile, although its uses are limited by side effects such as respiratory depression and neurotoxicity.
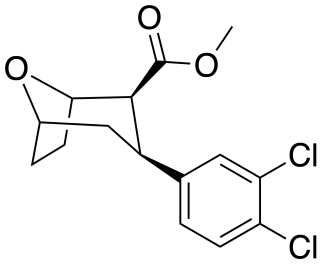
Tropoxane (O-1072) is an aryloxytropane derivative drug developed by Organix Inc., which acts as a stimulant and potent dopamine and serotonin reuptake inhibitor. It is an analogue of dichloropane where the amine nitrogen has been replaced by an oxygen ether link, demonstrating that the amine nitrogen is not required for DAT binding and reuptake inhibition.

Baicalein (5,6,7-trihydroxyflavone) is a flavone, a type of flavonoid, originally isolated from the roots of Scutellaria baicalensis and Scutellaria lateriflora. It is also a constituent of Oroxylum indicum and thyme. It is the aglycone of baicalin.
Cāng zhú, also known as black atractylodes rhizome or Rhizoma Atractylodes, is a Chinese herbal medicine. It is the dried rhizome of Atractylodes lancea (Thunb.) DC., synonyms Atractylodes chinensis (DC.) Koidz, and Atractylodes japonica Koidz. The medicine is distinguished from bái zhú, which is typically cultivated, whereas cāng zhú more often tends to be collected from the wild. It is believed that the distinction between cāng zhú and bái zhú emerged in relatively modern times; a single drug "zhú" described in the Shen nong ben cao jing probably included many Atractylodes species.
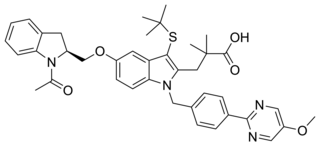
AM-679 is a drug which acts as a selective inhibitor of 5-Lipoxygenase-activating protein (FLAP). This protein is involved in the production of cysteinyl leukotrienes which are involved in inflammation, and AM-679 has anti-inflammatory effects in animal studies.
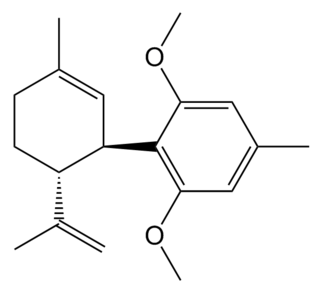
O-1918 is a synthetic compound related to cannabidiol, which is an antagonist at two former orphan receptors GPR18 and GPR55, that appear to be related to the cannabinoid receptors. O-1918 is used in the study of these receptors, which have been found to be targets for a number of endogenous and synthetic cannabinoid compounds, and are thought to be responsible for most of the non-CB1, non-CB2 mediated effects that have become evident in the course of cannabinoid research.
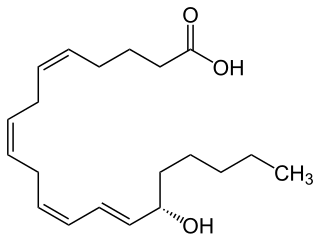
15-Hydroxyeicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HpETE). This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5(S),15(S)-dihydroxy-eicosatetraenoic acid (5(S),15(S)-diHETE), 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE), a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15(S)-HETE and 15(S)-HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.
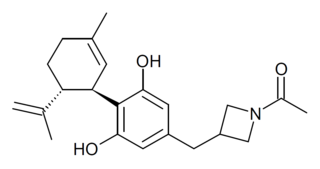
KLS-13019 is a cannabidiol derivative that has been modified on the side chain to improve solubility and tissue penetration properties. It was developed and patented by Neuropathix subsidiary Kannalife and found to be 50x more potent than cannabidiol as a neuroprotective agent, thought to be mediated by modulation of the sodium-calcium exchanger channel. It also had a higher therapeutic index than cannabidiol. Both KLS-13019 and cannabidiol, prevented the development of CIPN, while only KLS-13019 uniquely reversed neuropathic pain from chemotherapy. KLS-13019 binds to fewer biological targets than cannabidiol and KLS-13019 may possess the unique ability to reverse addictive behaviour, an effect not observed with cannabidiol.

7-Hydroxycannabidiol (7-OH-CBD) is an active metabolite of cannabidiol, generated in the body from cannabidiol by the action of the enzyme CYP2C19. While methods have been developed for its synthetic production, and measurement of levels in the body following consumption of cannabidiol, its pharmacology has been relatively little studied, though it has been found to possess similar anticonvulsant effects to cannabidiol itself, as well as lowering blood triglyceride levels. Like its precursor CBD, it is not known to exhibit any psychoactive effects on the body and is known to counter the psychoactive effects of THC if it is present at the same time. This mode of action in 2015 was discovered to be at least contributing in part by being a non competitive negative allosteric modulator of the Cannabinoid receptor type 1.


















