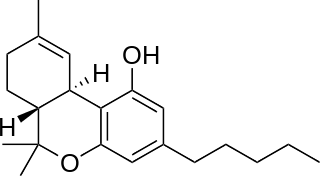
Tetrahydrocannabinol (THC) is a cannabinoid found in cannabis. It is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term THC usually refers to the delta-9-THC isomer with chemical name (−)-trans-Δ9-tetrahydrocannabinol. It is a colorless oil.

Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 100 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Tetrahydrocannabivarin is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of pentyl (5-carbon), making it non-psychoactive in lower doses. It has been shown to exhibit neuroprotective activity, appetite suppression, glycemic control and reduced side effects compared to THC, making it a potential treatment for management of obesity and diabetes. THCV was studied by Roger Adams as early as 1942.

HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended duration of action. HU-210 has a binding affinity of 0.061 nM at CB1 receptors compared to 40.7 nM for Δ9-THC. The binding pose of HU-210 to the CB1 receptor is similar to other synthetic cannabinoids.

Fosfluconazole is a water-soluble phosphate prodrug of fluconazole — a triazole antifungal drug used in the treatment and prevention of superficial and systemic fungal infections.

Parahexyl, also known as synhexyl, is a synthetic homologue of tetrahydrocannabinol (THC) which was invented in 1941 during attempts to elucidate the structure of Δ9-THC, one of the active components of cannabis.
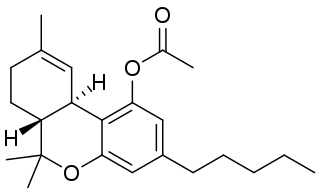
THC-O-acetate is the acetate ester of THC. The term THC-O-acetate and its variations are commonly used for two types of the substance, dependent on which cannabinoid it is synthesized from. The difference between Δ8-THC and Δ9-THC is bond placement on the cyclohexene ring.

Dimethylheptylpyran (DMHP) is a synthetic cannabinoid and analogue of tetrahydrocannabinol (THC). It was invented in 1949 during attempts to elucidate the structure of Δ9-THC, one of the active components of cannabis. DMHP is a pale yellow, viscous oil which is insoluble in water but dissolves in alcohol or non-polar solvents.
An active metabolite, or pharmacologically active metabolite is a biologically active metabolite of a xenobiotic substance, such as a drug or environmental chemical. Active metabolites may produce therapeutic effects, as well as harmful effects.

Fospropofol (INN), often used as the disodium salt is an intravenous sedative-hypnotic agent. It is currently approved for use in sedation of adult patients undergoing diagnostic or therapeutic procedures such as endoscopy.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.

Δ-3-Tetrahydrocannabinol is a synthetic isomer of tetrahydrocannabinol (THC) developed during the original research in the 1940s to develop synthetic routes to the natural products Δ8-THC and Δ9-THC found in the cannabis. While the normal trans configuration of THC is in this case flattened by the double bond, it still has two enantiomers as the 9-methyl group can exist in an (R) or (S) conformation. The (S) enantiomer has similar effects to Δ9-THC though with several times lower potency, while the (R) enantiomer is many times less active or inactive, depending on the assay used. It has been identified as a component of vaping liquid products.

Δ-10-Tetrahydrocannabinol is a positional isomer of tetrahydrocannabinol, discovered in the 1980s. Two epimers have been reported in the literature, with the 9-methyl group in either the (R) or (S) conformation; of these, the (R) epimer appears to be the more active isomer as well as the double bond in the 10th position instead of the 9th maintaining about 30 to 40 percent the potency of delta-9-THC. Δ10-THC has rarely been reported as a trace component of natural cannabis, though it is thought to be a degradation product similar to cannabinol rather than being produced by the plant directly. However, it is found more commonly as an impurity in synthetic delta-8-THC produced from cannabidiol and can also be synthesized directly from delta-9-THC.

THC morpholinylbutyrate is a synthetic derivative of tetrahydrocannabinol, developed in the 1970s. It is a prodrug which is converted into THC inside the body, and was one of the first derivatives of THC that is able to form water-soluble salts, giving it a significant advantage over THC for some applications. However, it is less potent than THC and the metabolic conversion to THC is relatively slow and variable, giving it unpredictable pharmacokinetics which has limited its research applications.

THC hemisuccinate is a synthetic derivative of tetrahydrocannabinol, developed in the 1990s. It is a water-soluble prodrug ester which is converted into THC inside the body, and was developed to overcome the poor bioavailability of THC when taken by non-inhaled routes of administration. In medical applications it has mainly been formulated as rectal suppositories.

11-Hydroxy-Δ-8-tetrahydrocannabinol is an active metabolite of Δ8-THC, a psychoactive cannabinoid found in small amounts in cannabis. It is an isomer of 11-OH-Δ9-THC, and is produced via the same metabolic pathway. It was the first cannabinoid metabolite discovered in 1970.

3'-Hydroxy-THC (3'-OH-Δ9-THC) is a minor active metabolite of THC, the main psychoactive component of cannabis. It is one of a number of metabolites of THC hydroxylated on the pentyl side chain, but while the other side-chain hydroxyl isomers are much weaker or inactive, the S enantiomer of 3'-OH-THC is several times more potent than THC itself, and while it is produced in smaller amounts than other active metabolites such as 11-Hydroxy-THC and 8,11-Dihydroxy-THC, it is thought to contribute to the overall pharmacological profile of cannabis.

11-Hydroxyhexahydrocannabinol is an active metabolite of tetrahydrocannabinol (THC) and a metabolite of the trace cannabinoid hexahydrocannabinol (HHC).
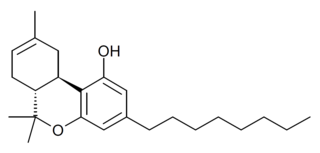
JWH-138 (THC-Octyl, Δ8-THC-C8) is a synthetic cannabinoid first synthesized by Roger Adams and studied heavily by John W. Huffman, with a Ki of 8.5nM at the CB1 cannabinoid receptor. THC-Octyl and its hydrogenated analog HHC-Octyl was synthesized and studied by Roger Adams as early as 1942.

THC valine hemisuccinate is a synthetic prodrug of tetrahydrocannabinol, developed at the University of Mississippi as a stabilised formulation for ophthalmic administration, for use in the treatment of glaucoma and other eye conditions requiring reduction in intraocular pressure.



















