
O-1057 is an analgesic cannabinoid derivative created by Organix Inc., Newburyport, Massachusetts, for use in scientific research. Unlike most cannabinoids discovered to date, it is water-soluble, which gives it considerable advantages over many related cannabinoids. It has moderate affinity for both CB1 and CB2 receptors, with Ki values of 8.36 nM at CB1 and 7.95 nM at CB2

JWH-051 is an analgesic drug which is a cannabinoid agonist. Its chemical structure is closely related to that of the potent cannabinoid agonist HU-210, with the only difference being the removal of the hydroxyl group at position 1 of the aromatic ring. It was discovered and named after John W. Huffman.

AMG-36 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC substituted with a cyclopentane group on the 3-position side chain. AMG-36 is a potent agonist at both CB1 and CB2 with moderate selectivity for CB1, with a Ki of 0.45 nM at CB1 vs 1.92 nM at CB2.
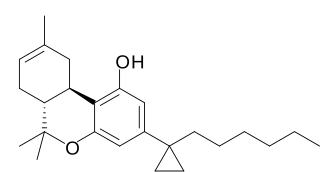
AMG-41 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC substituted with a cyclopropyl group on the C1'-position of the C3-alkyl side chain. AMG-41 is a potent agonist at both CB1 and CB2, with a Ki of 0.44 nM at CB1 vs 0.86 nM at CB2.

AM-906 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is conformationally restricted by virtue of the double bond on its side chain, leading an increased affinity for and selectivity between CB1 and CB2 receptors. It is a potent and selective agonist for the CB1 cannabinoid receptor, with a Ki of 0.8 nM at CB1 and 9.5 nM at CB2, a selectivity of almost 12x.

AM-905 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is conformationally restricted by virtue of the double bond on its side chain, leading an increased affinity for and selectivity between CB1 and CB2 receptors. It is a potent and reasonably selective agonist for the CB1 cannabinoid receptor, with a Ki of 1.2 nM at CB1 and 5.3 nM at CB2.

AMG-1 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC with a rigidified and extended 3-position side chain. AMG-1 is a potent agonist at both CB1 and CB2 with moderate selectivity for CB1, with a Ki of 0.6 nM at CB1 vs 3.1 nM at CB2.
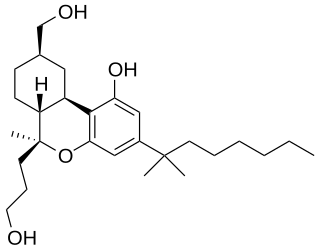
AM-919 is an analgesic drug which is a cannabinoid receptor agonist. It is a derivative of HU-210 which has been substituted with a 6β-(3-hydroxypropyl) group. This adds a "southern" aliphatic hydroxyl group to the molecule as seen in the CP-series of nonclassical cannabinoid drugs, and so AM-919 represents a hybrid structure between the classical dibenzopyran and nonclassical cannabinoid families.

AM-938 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid receptor agonist. It is a derivative of HU-210 which has been substituted with a 6β-(3-hydroxyprop-1-ynyl) group. This adds a "southern" aliphatic hydroxyl group to the molecule as seen in the CP-series of nonclassical cannabinoid drugs, and so AM-938 represents a hybrid structure between the classical and nonclassical cannabinoid families, with the 6-hydroxyalkyl chain rigidified with a triple bond. This gives AM-938 a greater degree of selectivity, so while it is still a potent agonist at both CB1 and CB2, it is reasonably selective for CB2, with a Ki of 0.3 nM at CB2 and 1.2 nM at CB1, a selectivity of around four-fold.

AM-4030 is an analgesic drug which is a cannabinoid receptor agonist. It is a derivative of HU-210 which has been substituted with a 6β-((E)-3-hydroxyprop-1-enyl) group. This adds a "southern" aliphatic hydroxyl group to the molecule as seen in the CP-series of nonclassical cannabinoid drugs, and so AM-4030 represents a hybrid structure between the classical and nonclassical cannabinoid families, with the 6-hydroxyalkyl chain rigidified with a double bond with defined stereochemistry. This gives AM-4030 a greater degree of selectivity, so while it is still a potent agonist at both CB1 and CB2, it is reasonably selective for CB1, with a Ki of 0.7nM at CB1 and 8.6nM at CB2, a selectivity of around 12x. Resolution of the enantiomers of AM-4030 yields an even more potent compound, although with less selectivity, with the (−) enantiomer AM-4030a having a Ki of 0.6nM at CB1 and 1.1nM at CB2.

CB-13 (CRA13, SAB-378) is a cannabinoid drug, which acts as a potent agonist at both the CB1 and CB2 receptors, but has poor blood–brain barrier penetration, and so produces only peripheral effects at low doses, with symptoms of central effects such as catalepsy only appearing at much higher dose ranges. It has antihyperalgesic properties in animal studies, and has progressed to preliminary human trials.

AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) is a designer drug that acts as a potent and selective agonist for the cannabinoid receptor CB1. It is used in scientific research for mapping the distribution of CB1 receptors.

A-834,735 is a drug developed by Abbott Laboratories that acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors, with a Ki of 12 nM at CB1 and 0.21 nM at CB2. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group of A-834,735 and related compounds imparts significant selectivity for CB2, with most compounds from this group found to be highly selective CB2 agonists with little affinity for CB1. However, low nanomolar CB1 binding affinity is retained with certain heterocyclic 1-position substituents such as (N-methylpiperidin-2-yl)methyl (cf. AM-1220, AM-1248), or the (tetrahydropyran-4-yl)methyl substituent of A-834,735, resulting in compounds that still show significant affinity and efficacy at both receptors despite being CB2 selective overall.

AM-1221 is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 0.28 nM at CB2 and 52.3 nM at the CB1 receptor, giving it around 180 times selectivity for CB2. The 2-methyl and 6-nitro groups on the indole ring both tend to increase CB2 affinity while generally reducing affinity at CB1, explaining the high CB2 selectivity of AM-1221. However, despite this relatively high selectivity for CB2, its CB1 affinity is still too strong to make it useful as a truly selective CB2 agonist, so the related compound AM-1241 is generally preferred for research purposes.
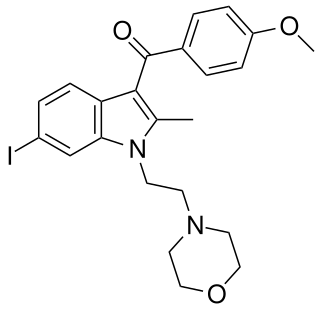
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.

AM-679 (part of the AM cannabinoid series) is a drug that acts as a moderately potent agonist for the cannabinoid receptors, with a Ki of 13.5 nM at CB1 and 49.5 nM at CB2. AM-679 was one of the first 3-(2-iodobenzoyl)indole derivatives that was found to have significant cannabinoid receptor affinity, and while AM-679 itself has only modest affinity for these receptors, it was subsequently used as a base to develop several more specialised cannabinoid ligands that are now widely used in research, including the potent CB1 agonists AM-694 and AM-2233, and the selective CB2 agonist AM-1241. AM-679 was first identified as having been sold as a cannabinoid designer drug in Hungary in 2011, along with another novel compound 1-pentyl-3-(1-adamantoyl)indole.

SR144528 is a drug that acts as a potent and highly selective CB2 receptor inverse agonist, with a Ki of 0.6 nM at CB2 and 400 nM at the related CB1 receptor. It is used in scientific research for investigating the function of the CB2 receptor, as well as for studying the effects of CB1 receptors in isolation, as few CB1 agonists that do not also show significant activity as CB2 agonists are available. It has also been found to be an inhibitor of sterol O-acyltransferase, an effect that appears to be independent from its action on CB2 receptors.

AM-2232 (1-(4-cyanobutyl)-3-(naphthalen-1-oyl)indole) is a drug that acts as a potent but unselective agonist for the cannabinoid receptors, with a Ki of 0.28 nM at CB1 and 1.48 nM at CB2.

AM-1235 (1-(5-fluoropentyl)-3-(naphthalen-1-oyl)-6-nitroindole) is a drug that acts as a potent and reasonably selective agonist for the cannabinoid receptor CB1.

AM-2389 is a classical cannabinoid derivative which acts as a potent and reasonably selective agonist for the CB1 receptor, with a Ki of 0.16 nM, and 26× selectivity over the related CB2 receptor. It has high potency in animal tests of cannabinoid activity, and a medium duration of action. Replacing the 1',1'-dimethyl substitution of the dimethylheptyl side chain of classical cannabinoids with cyclopropyl or cyclopentyl results in higher potency than cyclobutyl, but only the cyclobutyl derivatives show selectivity for CB1 over CB2. High selectivity for CB1 over CB2 is difficult to achieve (cf. AM-906, AM-1235), as almost all commonly used CB1 agonists have similar or greater affinity for CB2 than CB1, and the only truly highly selective CB1 agonists known as of 2012 are eicosanoid derivatives such as O-1812.




















