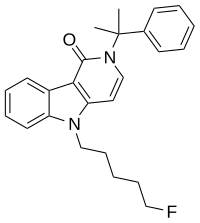
MN 18 is an indazole-based synthetic cannabinoid that is an agonist for the cannabinoid receptors, with Ki values of 45.72 nM at CB1 and 11.098 nM at CB2 and EC50 values of 2.028 nM at CB1 and 1.233 nM at CB2, and has been sold online as a designer drug. It is the indazole core analogue of NNE1. Given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, it is suspected that metabolic hydrolysis of the amide group of MN-18 may release 1-naphthylamine, a known carcinogen. MN-18 metabolism has been described in literature.

PB-22 is a designer drug offered by online vendors as a cannabimimetic agent, and detected being sold in synthetic cannabis products in Japan in 2013. PB-22 represents a structurally unique synthetic cannabinoid chemotype, since it contains an ester linker at the indole 3-position, rather than the precedented ketone of JWH-018 and its analogs, or the amide of APICA and its analogs.

QUCHIC is a designer drug offered by online vendors as a cannabimimetic agent, and was first detected being sold in synthetic cannabis products in Japan in early 2013, and subsequently also in New Zealand. The structure of QUCHIC appears to use an understanding of structure-activity relationships within the indole class of cannabimimetics, although its design origins are unclear. QUCHIC, along with QUPIC, represents a structurally unique synthetic cannabinoid chemotype since it contains an ester linker at the indole 3-position rather than the precedented ketone of JWH-018 and its analogues, or the amide of SDB-001 and its analogues.

SDB-006 is a drug that acts as a potent agonist for the cannabinoid receptors, with an EC50 of 19 nM for human CB2 receptors, and 134 nM for human CB1 receptors. It was discovered during research into the related compound SDB-001 which had been sold illicitly as "2NE1". SDB-006 metabolism has been described in literature.
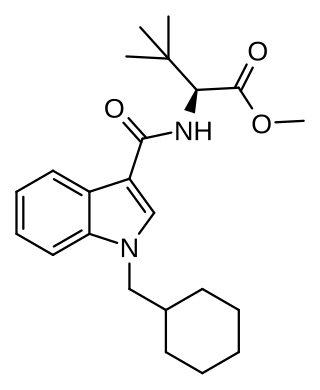
'MDMB-CHMICAa' is an indole-based synthetic cannabinoid that is a potent agonist of the CB1 receptor and has been sold online as a designer drug. While MDMB-CHMICA was initially sold under the name "MMB-CHMINACA", the compound corresponding to this code name (i.e. the isopropyl instead of t-butyl analogue of MDMB-CHMINACA) has been identified on the designer drug market in 2015 as AMB-CHMINACA.

5F-APINACA is an indazole-based synthetic cannabinoid that has been sold online as a designer drug. Structurally it closely resembles cannabinoid compounds from patent WO 2003/035005 but with a 5-fluoropentyl chain on the indazole 1-position, and 5F-APINACA falls within the claims of this patent, as despite not being disclosed as an example, it is very similar to the corresponding pentanenitrile and 4-chlorobutyl compounds which are claimed as examples 3 and 4.
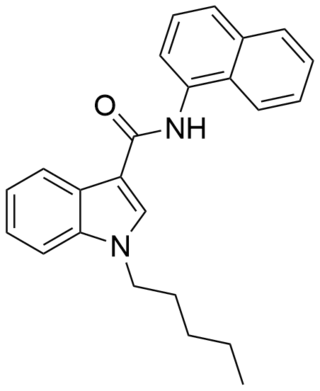
NNE1 (also known as NNEI, MN-24 and AM-6527) is an indole-based synthetic cannabinoid, representing a molecular hybrid of APICA and JWH-018 that is an agonist for the cannabinoid receptors, with Ki values of 60.09 nM at CB1 and 45.298 nM at CB2 and EC50 values of 9.481 nM at CB1 and 1.008 nM at CB2. It was invented by Abbott and has a CB1 receptor pEC50 of 8.9 with around 80x selectivity over the related CB2 receptor. It is suspected that metabolic hydrolysis of the amide group of NNE1 may release 1-naphthylamine, a known carcinogen, given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, and NNE1 was banned in New Zealand in 2012 as a temporary class drug to stop it being used as an ingredient in then-legal synthetic cannabis products. NNE1 was subsequently found to be responsible for the death of a man in Japan in 2014.

NM-2201 (also known as CBL-2201 and NA-5F-PIC) is an indole-based synthetic cannabinoid that presumably has similar properties to the closely related 5F-PB-22 and NNE1, which are both full agonists and unselectively bind to CB1 and CB2 receptors with low nanomolar affinity.

5F-CUMYL-PINACA (also known as SGT-25 and sometimes sold in e-cigarette form as C-Liquid) is an indazole-3-carboxamide based synthetic cannabinoid. 5F-CUMYL-PINACA acts as a potent agonist for the cannabinoid receptors, with the original patent claiming approximately 4x selectivity for CB1, having an EC50 of <0.1 nM for human CB1 receptors and 0.37 nM for human CB2 receptors. In more recent assays using different techniques, 5F-CUMYL-PINACA was variously found to have an EC50 of 0.43 nM at CB1 and 11.3 nM at CB2, suggesting a somewhat higher CB1 selectivity of 26 times, or alternatively 15.1 nM at CB1 and 34.8 nM at CB2 with only 2.3 times selectivity, however these figures cannot be directly compared due to the different assay techniques used in each case.
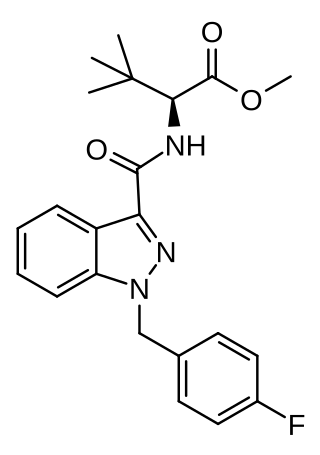
MDMB-FUBINACA (also known as MDMB(N)-Bz-F and FUB-MDMB) is an indazole-based synthetic cannabinoid that is a potent agonist for the cannabinoid receptors, with Ki values of 1.14 nM at CB1 and 0.1228 nM at CB2 and EC50 values of 0.2668 nM at CB1 and 0.1411 nM at CB2, and has been sold online as a designer drug. Its benzyl analogue (instead of 4-fluorobenzyl) has been reported to be a potent agonist for the CB1 receptor (Ki = 0.14 nM, EC50 = 2.42 nM). The structure of MDMB-FUBINACA contains the amino acid, 3-methylvaline or tert-leucine methyl ester.
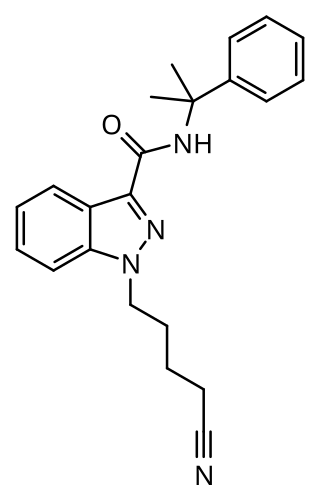
CUMYL-4CN-BINACA (also known as CUMYL-CYBINACA or SGT-78) is an indazole-3-carboxamide based synthetic cannabinoid that has been sold online as a designer drug. It is a potent agonist for cannabinoid receptors CB1 and CB2, with in vitro EC50 values of 0.58 nM and 6.12 nM, respectively. In mice, CUMYL-4CN-BINACA produces hypothermic and pro-convulsant effects via the CB1 receptor, and anecdotal reports suggest it has an active dose of around 0.1 mg in humans.
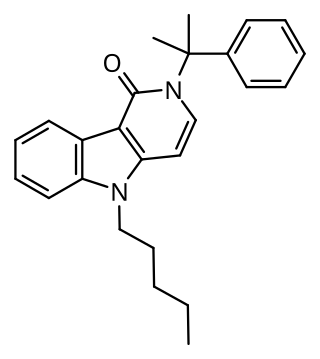
CUMYL-PEGACLONE (SGT-151) is a gamma-carboline based synthetic cannabinoid that has been sold as a designer drug. The gamma-carboline core structure seen in CUMYL-PEGACLONE had not previously been encountered in a designer cannabinoid, though it is similar in structure to other gamma-carboline cannabinoids disclosed by Bristol-Myers Squibb in 2001.

5F-MDMB-PICA (MDMB-5F-PICA) is a designer drug and synthetic cannabinoid. In 2018, it was the fifth-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Administration.

4F-MDMB-BINACA (also known as MDMB-4F-BINACA, 4F-MDMB-BUTINACA or 4F-ADB) is an indazole-based synthetic cannabinoid from the indazole-3-carboxamide family. It should not be confused with the amantadine analogue 4F-ABINACA. It has been used as an active ingredient in synthetic cannabis products and sold as a designer drug since late 2018. 4F-MDMB-BINACA is an agonist of the CB1 receptor (EC50 = 7.39 nM), though it is unclear whether it is selective for this target. In December 2019, the UNODC announced scheduling recommendations placing 4F-MDMB-BINACA into Schedule II throughout the world.
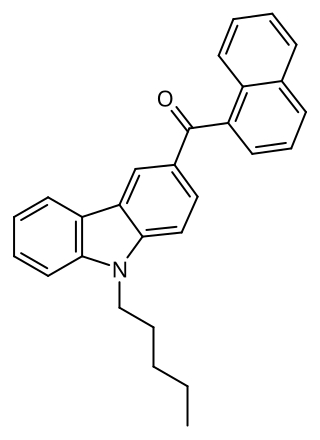
EG-018 is a carbazole-based synthetic cannabinoid that has been sold online as a designer drug. It acts as a partial agonist of the CB1 and CB2 receptor, with reasonably high binding affinity, but low efficacy in terms of inducing a signaling response.

CUMYL-CH-MEGACLONE is a gamma-carboline based synthetic cannabinoid receptor agonist that has been sold as a designer drug, first being identified in Hungary in December 2018.

CUMYL-CBMICA (SGT-280) is an indole-3-carboxamide based synthetic cannabinoid receptor agonist which has been sold as a designer drug, first being identified in Germany in August 2019. Since the structure fell outside the German drug analogue law provisions at the time, an amendment was made to the law to expand the relevant definition, which came into effect in April 2020. It has been shown to act as a CB1 receptor agonist with an EC50 of 62.9nM.

CUMYL-FUBINACA (SGT-149) is an indazole-3-carboxamide based synthetic cannabinoid receptor agonist, with an EC50 of 1.8nM for human CB1 receptors and 23.7nM for human CB2 receptors, giving it around 13x selectivity for CB1. It has been sold online as a designer drug.

ADB-BINACA (also known as ADMB-BZINACA using EMCDDA naming standards) is a cannabinoid designer drug that has been found as an ingredient in some synthetic cannabis products. It was originally developed by Pfizer as a potential analgesic, and is a potent agonist of the CB1 receptor with a binding affinity (Ki) of 0.33 nM and an EC50 of 14.7 nM.

ADB-BUTINACA (also known as ADMB-BINACA using EMCDDA naming standards) is a synthetic cannabinoid compound which has been sold as a designer drug. It is a potent CB1 agonist, with a binding affinity of 0.29nM for CB1 and 0.91nM for CB2, and an EC50 of 6.36 nM for CB1.