
WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure.

The cannabinoid receptor 2(CB2), is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the CNR2 gene. It is closely related to the cannabinoid receptor 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids. The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG).
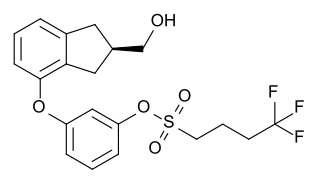
Originally synthesized by chemist Wayne E. Kenney, BAY 38-7271 (KN 38-7271) is a drug which is a cannabinoid receptor agonist developed by Bayer AG. It has analgesic and neuroprotective effects and is used in scientific research, with proposed uses in the treatment of traumatic brain injury. It is a full agonist with around the same potency as CP 55,940 in animal studies, and has fairly high affinity for both CB1 and CB2 receptors, with Ki values of 2.91nM at CB1 and 4.24nM at CB2. It has been licensed to KeyNeurotek Pharmaceuticals for clinical development, and was in Phase II trials in 2008 but its development appears to have stopped.

JWH-051 is an analgesic drug which is a cannabinoid agonist. Its chemical structure is closely related to that of the potent cannabinoid agonist HU-210, with the only difference being the removal of the hydroxyl group at position 1 of the aromatic ring. It was discovered and named after John W. Huffman.

GW-405,833 (L-768,242) is a drug that acts as a potent and selective partial agonist for the cannabinoid receptor subtype CB2, with an EC50 of 0.65 nM and selectivity of around 1200x for CB2 over CB1 receptors. Animal studies have shown it to possess antiinflammatory and anti-hyperalgesic effects at low doses, followed by ataxia and analgesic effects when the dose is increased. Selective CB2 agonist drugs such as GW-405,833 are hoped to be particularly useful in the treatment of allodynia and neuropathic pain for which current treatment options are often inadequate.

AM-1241 (1-(methylpiperidin-2-ylmethyl)-3-(2-iodo-5-nitrobenzoyl)indole) is a chemical from the aminoalkylindole family that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 3.4 nM at CB2 and 80 times selectivity over the related CB1 receptor. It has analgesic effects in animal studies, particularly against "atypical" pain such as hyperalgesia and allodynia. This is thought to be mediated through CB2-mediated peripheral release of endogenous opioid peptides, as well as direct activation of the TRPA1 channel. It has also shown efficacy in the treatment of amyotrophic lateral sclerosis in animal models.
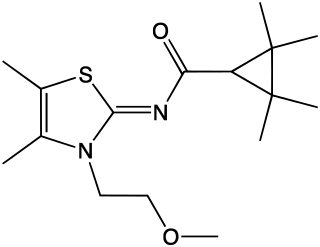
A-836,339 is a drug developed by Abbott Laboratories that acts as a potent cannabinoid receptor full agonist. It is selective for CB2, with Ki values of 0.64 nM at CB2 vs 270 nM at the psychoactive CB1 receptor, but while it exhibits selective analgesic, anti-inflammatory and anti-hyperalgesic effects at low doses, its high efficacy at both targets results in typical cannabis-like effects appearing at higher doses, despite its low binding affinity for CB1. In 2012 A-836,339 was detected via X-ray crystallography in a "dubious product" sold in Japan, though the product was described as a white powder, not herbal incense, it was suggested to be for human consumption.
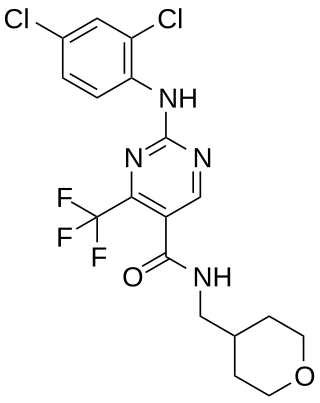
GW-842,166X is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist, with a novel chemical structure based on a pyrimidine core. It has potent analgesic, anti-inflammatory and anti-hyperalgesic actions in animal models, but without cannabis-like behavioural effects due to its extremely low affinity for the CB1 receptor. GSK brought this compound for into 4 clinical trials, two of them related to pain management and the other two related to bio-distributions. The trials were either withdrawn or completed without posting result.

A-796,260 is a drug developed by Abbott Laboratories that acts as a potent and selective cannabinoid CB2 receptor agonist. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group, imparts significant selectivity for CB2, and A-796,260 was found to be a highly selective CB2 agonist with little affinity for CB1, having a CB2Ki of 4.6 nM vs 945 nM at CB1. It has potent analgesic and anti-inflammatory actions in animal models, being especially effective in models of neuropathic pain, but without producing cannabis-like behavioral effects.

AM-1221 is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 0.28 nM at CB2 and 52.3 nM at the CB1 receptor, giving it around 180 times selectivity for CB2. The 2-methyl and 6-nitro groups on the indole ring both tend to increase CB2 affinity while generally reducing affinity at CB1, explaining the high CB2 selectivity of AM-1221. However, despite this relatively high selectivity for CB2, its CB1 affinity is still too strong to make it useful as a truly selective CB2 agonist, so the related compound AM-1241 is generally preferred for research purposes.

AZ-11713908 is a drug developed by AstraZeneca which is a peripherally selective cannabinoid agonist, acting as a potent agonist at the CB1 receptor and a partial agonist at CB2. It has poor blood–brain barrier penetration, and so while it is an effective analgesic in animal tests, it produces only peripheral effects at low doses, with much weaker symptoms of central effects compared to other cannabinoid drugs such as WIN 55,212-2. Many related benzimidazole-derived cannabinoid ligands are known.

MDA-19 (also known as BZO-HEXOXIZID) is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with reasonable selectivity over the psychoactive CB1 receptor, though with some variation between species. In animal studies it was effective for the treatment of neuropathic pain, but did not affect rat locomotor activity in that specific study. The pharmacology of MDA-19 in rat cannabinoid receptors have been demonstrated to function differently than human cannabinoid receptors with MDA-19 binding to human CB1 receptors 6.9× higher than rat CB1 receptors.

CBS-0550 is a drug developed by Taisho Pharmaceutical, which acts as a potent and selective cannabinoid CB2 receptor agonist, with 1400x selectivity for CB2 over the related CB1 receptor. Unlike most cannabinoid agonists, CBS-0550 has good solubility in water, and in animal studies it was found to produce analgesic and anti-hyperalgesic effects. A number of related compounds have been developed with similar properties.
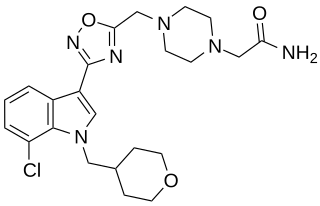
LBP-1 is a drug originally developed by Organon for the treatment of neuropathic pain, It acts as a potent and selective cannabinoid receptor agonist, with high potency at both the CB1 and CB2 receptors, but low penetration of the blood–brain barrier. This makes LBP-1 peripherally selective, and while it was effective in animal models of neuropathic pain and allodynia, it did not produce cannabinoid-appropriate responding suggestive of central effects, at any dose tested.

AM-1714 (part of the AM cannabinoid series) is a drug that acts as a reasonably selective agonist of the peripheral cannabinoid receptor CB2, with sub-nanomolar affinity and 490x selectivity over the related CB1 receptor. In animal studies it has both analgesic and anti-allodynia effects. The 9-methoxy derivative AM-1710 has similar CB2 affinity but only 54x selectivity over CB1.

Olorinab (APD371) is a drug being developed by Arena Pharmaceuticals for the treatment of gastrointestinal pain associated with Crohn's disease and irritable bowel syndrome. It acts as a potent and selective cannabinoid CB2 receptor agonist and is claimed to be orally active and peripherally selective. Initial Phase IIa exploratory clinical trials have been successful in patients with quiescent Crohn's disease. Arena initiated the Phase IIb Captivate trial in late July 2019 in patients with irritable bowel syndrome related pain, in constipation and diarrhea predominant sub-types. The Phase IIb trial is expected to enroll 240 participants between the ages of 18 and 70.Three doses of 10 mg, 25 mg, and 50 mg are being tested against Placebo in a 3:4 prescription ratio with a Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) masking layout.

AZD-1940 is a drug developed by AstraZeneca, that is a peripherally selective cannabinoid agonist which binds with high affinity to both the CB1 and CB2 receptors. It was developed for the treatment of neuropathic pain, but while it showed good peripheral selectivity in animal studies, in human clinical trials it failed to show sufficient analgesic efficacy and produced unexpectedly strong side effects associated with central cannabinoid activity, and so was discontinued from further development.

MCHB-1 is a benzimidazole derived drug which was researched as an analgesic but never developed for medical use. It acts as a potent agonist of the CB2 receptor, with an EC50 of 0.52nM at CB2, and ~30x selectivity over CB1 (Ki of 110nM at CB1 vs 3.7nM at CB2). It has been sold online as a designer drug, first being identified in Germany in December 2013.

Cannabinor (PRS-211,375) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist. It is classed as a "nonclassical" cannabinoid with a chemical structure similar to that of cannabidiol. It has a CB2 affinity of 17.4 nM vs 5,585 nM at CB1, giving it over 300× selectivity for CB2. It showed analgesic effects in animal studies especially in models of neuropathic pain, but failed in Phase IIb human clinical trials due to lack of efficacy.



















