
Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 100 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

O-2545 is an analgesic cannabinoid derivative created by Organix Inc. for use in scientific research. Unlike most cannabinoids discovered to date, it is water-soluble, which gives it considerable advantages over many related cannabinoids. It has high affinity for both CB1 and CB2 receptors, with Ki values of 1.5 nM at CB1 and 0.32 nM at CB2.

AMG-1 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC with a rigidified and extended 3-position side chain. AMG-1 is a potent agonist at both CB1 and CB2 with moderate selectivity for CB1, with a Ki of 0.6 nM at CB1 vs 3.1 nM at CB2.
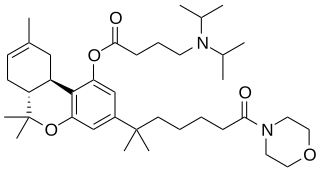
O-2694 is a drug that is a cannabinoid derivative. It has analgesic effects and is used in scientific research. Unlike most cannabinoids discovered to date, it is highly water-soluble, which gives it considerable advantages over many related drugs. It has high affinity for both CB1 and CB2 receptors, with Ki values of 3.7 nM at CB1 and 2.8 nM at CB2. However, it has complex pharmacokinetics as most of the administered dose is metabolised by hydrolysis of the ester link to the water-insoluble compound O-2372, thus producing a biphasic effects profile that is less suitable for research purposes than the related compound O-2545.
RVD-Hpα (pepcan-12) is an endogenous neuropeptide found in human and mammalian brain, which was originally proposed to act as a selective agonist for the CB1 cannabinoid receptor. It is a 12-amino acid polypeptide having the amino acid sequence Arg-Val-Asp-Pro-Val-Asn-Phe-Lys-Leu-Leu-Ser-His and is an N-terminal extended form of hemopressin, a 9-AA polypeptide derived from the α1 subunit of hemoglobin which has previously been shown to act as a CB1 inverse agonist. All three polypeptides have been isolated from various mammalian species, with RVD-Hpα being one of the more abundant neuropeptides expressed in mouse brain, and these neuropeptides represent a new avenue for cannabinoid research distinct from the previously known endogenous lipid-derived cannabinoid agonists such as anandamide. Recently it was shown that RVD-Hpα (also called Pepcan-12) is a potent negative allosteric modulator at CB1 receptors, together with other newly described N-terminally extended peptides (pepcans).

AM-1221 is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 0.28 nM at CB2 and 52.3 nM at the CB1 receptor, giving it around 180 times selectivity for CB2. The 2-methyl and 6-nitro groups on the indole ring both tend to increase CB2 affinity while generally reducing affinity at CB1, explaining the high CB2 selectivity of AM-1221. However, despite this relatively high selectivity for CB2, its CB1 affinity is still too strong to make it useful as a truly selective CB2 agonist, so the related compound AM-1241 is generally preferred for research purposes.
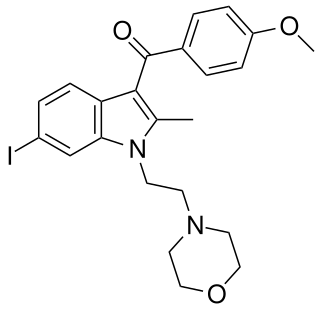
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.
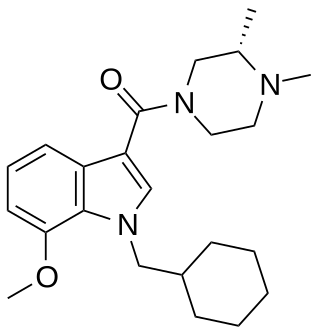
Org 28611 (SCH-900,111) is a drug developed by Organon International which acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors. It was developed with the aim of finding a water-soluble cannabinoid agonist suitable for intravenous use as an analgesic, and while it achieved this aim and has progressed as far as Phase II clinical trials in humans as both a sedative and an analgesic, results against the comparison drugs (midazolam and morphine respectively) were not particularly favourable in initial testing.

Org 28312 is a drug developed by Organon International which acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors. It was developed with the aim of finding a water-soluble cannabinoid agonist suitable for intravenous use as an analgesic, but did not proceed to human trials, with the related compound Org 28611 chosen instead due to its better penetration into the brain. The structure-activity relationships of these compounds have subsequently been investigated further leading to the development of a number of more potent analogues, derived by cyclisation around the indole or piperazine rings.

SR144528 is a drug that acts as a potent and highly selective CB2 receptor inverse agonist, with a Ki of 0.6 nM at CB2 and 400 nM at the related CB1 receptor. It is used in scientific research for investigating the function of the CB2 receptor, as well as for studying the effects of CB1 receptors in isolation, as few CB1 agonists that do not also show significant activity as CB2 agonists are available. It has also been found to be an inhibitor of sterol O-acyltransferase, an effect that appears to be independent from its action on CB2 receptors.

AM-2232 (1-(4-cyanobutyl)-3-(naphthalen-1-oyl)indole) is a drug that acts as a potent but unselective agonist for the cannabinoid receptors, with a Ki of 0.28 nM at CB1 and 1.48 nM at CB2.

AM-1235 (1-(5-fluoropentyl)-3-(naphthalen-1-oyl)-6-nitroindole) is a drug that acts as a potent and reasonably selective agonist for the cannabinoid receptor CB1.

O-1812 is an eicosanoid derivative related to anandamide that acts as a potent and highly selective agonist for the cannabinoid receptor CB1, with a Ki of 3.4 nM at CB1 and 3870 nM at CB2. Unlike most related compounds, O-1812 is metabolically stable against rapid breakdown by enzymes, and produces a cannabinoid-like discriminative effect in rats, which is similar but not identical to that produced by cannabinoid drugs of other chemical classes.

O-2372 is an analgesic cannabinoid derivative created by Organix Inc. for use in scientific research. It has high affinity for both CB1 and CB2 receptors, with Ki values of 1.3 nM at CB1 and 0.57 nM at CB2, but is only moderately soluble in water compared to other related compounds such as O-2694, which it is a metabolite of.
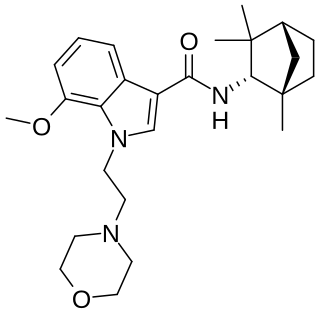
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

MDA-19 (also known as BZO-HEXOXIZID) is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with reasonable selectivity over the psychoactive CB1 receptor, though with some variation between species. In animal studies it was effective for the treatment of neuropathic pain, but did not affect rat locomotor activity in that specific study. The pharmacology of MDA-19 in rat cannabinoid receptors have been demonstrated to function differently than human cannabinoid receptors with MDA-19 binding to human CB1 receptors 6.9× higher than rat CB1 receptors.

AM-2389 is a classical cannabinoid derivative which acts as a potent and reasonably selective agonist for the CB1 receptor, with a Ki of 0.16 nM, and 26× selectivity over the related CB2 receptor. It has high potency in animal tests of cannabinoid activity, and a medium duration of action. Replacing the 1',1'-dimethyl substitution of the dimethylheptyl side chain of classical cannabinoids with cyclopropyl or cyclopentyl results in higher potency than cyclobutyl, but only the cyclobutyl derivatives show selectivity for CB1 over CB2. High selectivity for CB1 over CB2 is difficult to achieve (cf. AM-906, AM-1235), as almost all commonly used CB1 agonists have similar or greater affinity for CB2 than CB1, and the only truly highly selective CB1 agonists known as of 2012 are eicosanoid derivatives such as O-1812.

CBS-0550 is a drug developed by Taisho Pharmaceutical, which acts as a potent and selective cannabinoid CB2 receptor agonist, with 1400x selectivity for CB2 over the related CB1 receptor. Unlike most cannabinoid agonists, CBS-0550 has good solubility in water, and in animal studies it was found to produce analgesic and anti-hyperalgesic effects. A number of related compounds have been developed with similar properties.

Cannabinor (PRS-211,375) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist. It is classed as a "nonclassical" cannabinoid with a chemical structure similar to that of cannabidiol. It has a CB2 affinity of 17.4 nM vs 5,585 nM at CB1, giving it over 300× selectivity for CB2. It showed analgesic effects in animal studies especially in models of neuropathic pain, but failed in Phase IIb human clinical trials due to lack of efficacy.
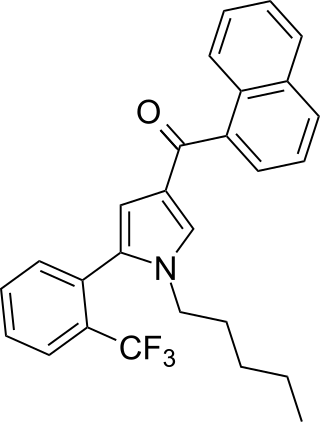
JWH-372 (naphthalen-1-yl-[1-pentyl-5-[2-(trifluoromethyl)phenyl]pyrrol-3-yl]methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as a potent and selective agonist of the CB2 receptor. JWH-372 binds approximately 9 times stronger to the CB2 receptor (Ki = 8.2 ± 0.2nM) than the CB1 receptor (Ki = 77 ± 2nM). The selectivity of JWH-372 for the CB2 receptor is likely due to the electron-withdrawing character of the trifluoromethyl group rather than steric effects, as the o-methyl compound JWH-370 was only mildly selective for the CB2 receptor (CB1 Ki = 5.6 ± 0.4nM, CB2 Ki = 4.0 ± 0.5nM).



















