Tetrahydrocannabinol (THC) is a cannabinoid found in cannabis. It is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term THC usually refers to the delta-9-THC isomer with chemical name (−)-trans-Δ9-tetrahydrocannabinol. It is a colorless oil.

Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 100 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.
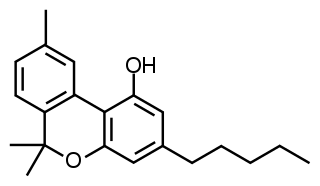
Cannabinol (CBN) is a mildly psychoactive phytocannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS).
A drug with psychotomimetic actions mimics the symptoms of psychosis, including delusions and/or delirium, as opposed to only hallucinations. Psychotomimesis is the onset of psychotic symptoms following the administration of such a drug.
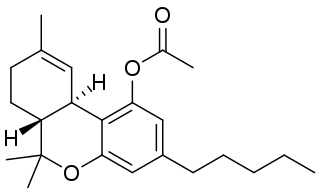
THC-O-acetate is the acetate ester of THC. The term THC-O-acetate and its variations are commonly used for two types of the substance, dependent on which cannabinoid it is synthesized from. The difference between Δ8-THC and Δ9-THC is bond placement on the cyclohexene ring.

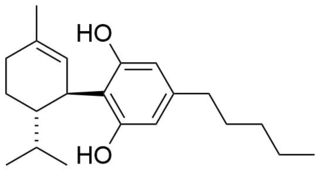
Cannabigerol (CBG) is a non-psychoactive cannabinoid and minor constituent of cannabis. It is one of more than 120 identified cannabinoids found in the plant genus Cannabis. The compound is the decarboxylated form of cannabigerolic acid (CBGA), the parent molecule from which other cannabinoids are biosynthesized.
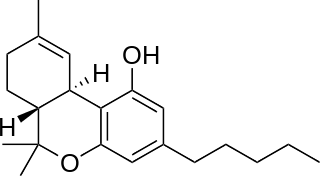
Δ9-Tetrahydrocannabutol is a phytocannabinoid found in cannabis that is a homologue of tetrahydrocannabinol (THC), the main active component of Cannabis. Structurally, they are only different by the pentyl side chain being replaced by a butyl side chain. THCB was studied by Roger Adams as early as 1942

11-Nor-9-carboxy-Δ9-tetrahydrocannabinol, often referred to as 11-nor-9-carboxy-THC or THC-11-oic acid, is the main secondary metabolite of tetrahydrocannabinol (THC) which is formed in the body after cannabis is consumed.

Cannabichromene (CBC), also called cannabichrome, cannanbichromene, pentylcannabichromene or cannabinochromene, exhibits anti-inflammatory properties in vitro, which may, theoretically, contribute to cannabis analgesic effects. It is a phytocannabinoid, one of the hundreds of cannabinoids found in the Cannabis plant. It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol (THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabinol (CBN), among others. CBC and cannabinols are present in cannabis. It is not scheduled by the Convention on Psychotropic Substances.

Tetrahydrocannabinolic acid is a precursor of tetrahydrocannabinol (THC), an active component of cannabis.
8,9-Dihydrocannabidiol is a synthetic cannabinoid that is closely related to cannabidiol (CBD) itself. that was first synthesized by Alexander R. Todd in 1940 derived from the catalytic hydrogenation of cannabidiol.

Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 receptor agonist which was known as a synthetic homologue of tetrahydrocannabinol (THC), but for the first time in 2019 was isolated as a natural product in trace amounts from Cannabis sativa.

Δ-8-tetrahydrocannabinol is a psychoactive cannabinoid found in the cannabis plant. It is an isomer of delta-9-tetrahydrocannabinol, the compound commonly known as THC, with which it co-occurs in hemp; natural quantities of ∆8-THC found in hemp are low. Psychoactive effects are similar to that of Δ9-THC, with central effects occurring by binding to cannabinoid receptors found in various regions of the brain.

Δ-3-Tetrahydrocannabinol is a synthetic isomer of tetrahydrocannabinol (THC) developed during the original research in the 1940s to develop synthetic routes to the natural products Δ8-THC and Δ9-THC found in the cannabis. While the normal trans configuration of THC is in this case flattened by the double bond, it still has two enantiomers as the 9-methyl group can exist in an (R) or (S) conformation. The (S) enantiomer has similar effects to Δ9-THC though with several times lower potency, while the (R) enantiomer is many times less active or inactive, depending on the assay used. It has been identified as a component of vaping liquid products.

Δ-10-Tetrahydrocannabinol is a positional isomer of tetrahydrocannabinol, discovered in the 1980s. Two epimers have been reported in the literature, with the 9-methyl group in either the (R) or (S) conformation; of these, the (R) epimer appears to be the more active isomer as well as the double bond in the 10th position instead of the 9th maintaining about 30 to 40 percent the potency of delta-9-THC. Δ10-THC has rarely been reported as a trace component of natural cannabis, though it is thought to be a degradation product similar to cannabinol rather than being produced by the plant directly. However, it is found more commonly as an impurity in synthetic delta-8-THC produced from cannabidiol and can also be synthesized directly from delta-9-THC.

Hexahydrocannabinol (HHC) is a hydrogenated derivative of tetrahydrocannabinol (THC). It is a naturally occurring phytocannabinoid that has rarely been identified as a trace component in Cannabis sativa, but can also be produced synthetically by firstly acid cyclization of cannabidiol and then hydrogenation of tetrahydrocannabinol. The synthesis and bioactivity of HHC was first reported in 1940 by Roger Adams.

Δ-6-cannabidiol is a positional isomer of cannabidiol, found in only trace amounts in natural cannabis plants but readily synthesised from cannabidiol by base-catalysed migration of the double bond.

Cannabitriol (CBT) is a phytocannabinoid first isolated in 1966, an oxidation product of tetrahydrocannabinol (THC) which has been identified both as a trace component of cannabis and as a metabolite in cannabis users. Its pharmacology has been little studied, though it has been found to act as an antiestrogen and aromatase inhibitor.

Tetrahydrocannabihexol is a phytocannabinoid, the hexyl homologue of tetrahydrocannabinol (THC) which was first isolated from Cannabis plant material in 2020 along with the corresponding hexyl homologue of cannabidiol, though it had been known for several decades prior to this as an isomer of the synthetic cannabinoid parahexyl. Another isomer Δ8-THCH is also known as a synthetic cannabinoid under the code number JWH-124, though it is unclear whether this occurs naturally in Cannabis, but likely is due to Δ8-THC itself being a degraded form of Δ9-THC. THC-Hexyl can be synthesized from 4-hexylresorcinol and was studied by Roger Adams as early as 1942.
Cannabinoids are compounds found in the cannabis plant or synthetic compounds that can interact with the endocannabinoid system. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (Delta-9-THC), the primary intoxicating compound in cannabis. Cannabidiol (CBD) is another major constituent of some cannabis plants. Conversion of CBD to THC can occur when CBD is heated to temperatures between 250–300 °C, potentially leading to its partial transformation into THC.


















