
MN 18 is an indazole-based synthetic cannabinoid that is an agonist for the cannabinoid receptors, with Ki values of 45.72 nM at CB1 and 11.098 nM at CB2 and EC50 values of 2.028 nM at CB1 and 1.233 nM at CB2, and has been sold online as a designer drug. It is the indazole core analogue of NNE1. Given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, it is suspected that metabolic hydrolysis of the amide group of MN-18 may release 1-naphthylamine, a known carcinogen. MN-18 metabolism has been described in literature.
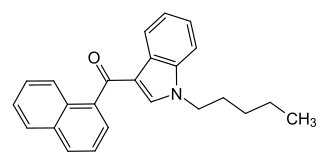
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in animals similar to those of tetrahydrocannabinol (THC), a cannabinoid naturally present in cannabis, leading to its use as synthetic cannabinoid products that, in some countries, are sold legally as "incense blends".

AM-2201 is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor. It is part of the AM series of cannabinoids discovered by Alexandros Makriyannis at Northeastern University.

AB-001 (1-pentyl-3-(1-adamantoyl)indole) is a designer drug that was found as an ingredient in synthetic cannabis smoking blends in Ireland in 2010 and Hungary and Germany in 2011. It is unclear who AB-001 was originally developed by, but it is structurally related to compounds such as AM-1248 and its corresponding 1-(tetrahydropyran-4-ylmethyl) analogue, which are known to be potent cannabinoid agonists with moderate to a high selectivity for CB2 over CB1. The first published synthesis and pharmacological evaluation of AB-001 revealed that it acts as a full agonist at CB1 (EC50 = 35 nM) and CB2 receptors (EC50 = 48 nM). However, AB-001 was found to possess only weak cannabimimetic effects in rats at doses up to 30 mg/kg, making it less potent than the carboxamide analogue APICA, which possesses potent cannabimimetic activity at doses of 3 mg/kg.

APINACA (AKB48, N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide) is a drug that acts as a reasonably potent agonist for the cannabinoid receptors. It is a full agonist at CB1 with an EC50 of 142 nM and Ki of 3.24 nM (compared to the Ki of Δ9-THC at 28.35 nM and JWH-018 at 9.62 nM), while at CB2 it acts as a partial agonist with an EC50 of 141 nM and Ki of 1.68 nM (compared to the Ki of Δ9-THC at 37.82 nM and JWH-018 at 8.55 nM). Its pharmacological characterization has also been reported in a discontinued patent application. It had never previously been reported in the scientific or patent literature, and was first identified by laboratories in Japan in March 2012 as an ingredient in synthetic cannabis smoking blends, along with a related compound APICA. Structurally, it closely resembles cannabinoid compounds from a University of Connecticut patent, but with a simple pentyl chain on the indazole 1-position, and APINACA falls within the claims of this patent despite not being disclosed as an example.

APICA is an indole based drug that acts as a potent agonist for the cannabinoid receptors.

STS-135 (N-(adamantan-1-yl)-1-(5-fluoropentyl)-1H-indole-3-carboxamide, also called 5F-APICA) is a designer drug offered by online vendors as a cannabimimetic agent. The structure of STS-135 appears to use an understanding of structure-activity relationships within the indole class of cannabimimetics, although its design origins are unclear. STS-135 is the terminally-fluorinated analogue of SDB-001, just as AM-2201 is the terminally-fluorinated analogue of JWH-018, and XLR-11 is the terminally-fluorinated analogue of UR-144. STS-135 acts a potent cannabinoid receptor agonist in vitro, with an EC50 of 51 nM for human CB2 receptors, and 13 nM for human CB1 receptors. STS-135 produces bradycardia and hypothermia in rats at doses of 1–10 mg/kg, suggesting cannabinoid-like activity.

PB-22 is a designer drug offered by online vendors as a cannabimimetic agent, and detected being sold in synthetic cannabis products in Japan in 2013. PB-22 represents a structurally unique synthetic cannabinoid chemotype, since it contains an ester linker at the indole 3-position, rather than the precedented ketone of JWH-018 and its analogs, or the amide of APICA and its analogs.

5F-PB-22 is a designer drug which acts as a cannabinoid agonist. The structure of 5F-PB-22 appears to have been designed with an understanding of structure–activity relationships within the indole class of cannabinoids.

ADBICA (also known as ADB-PICA) is a designer drug identified in synthetic cannabis blends in Japan in 2013. ADBICA had not previously been reported in the scientific literature prior to its sale as a component of synthetic cannabis blends. ADBICA features a carboxamide group at the 3-indole position, like SDB-001 and STS-135. The stereochemistry of the tert-butyl side-chain in the product is unresolved, though in a large series of indazole derivatives structurally similar to ADBICA that are disclosed in Pfizer patent WO 2009/106980, activity resides exclusively in the (S) enantiomers. ADBICA is a potent agonist of the CB1 receptor and CB2 receptor with an EC50 value of 0.69 nM and 1.8 nM respectively.

ADB-FUBINACA (ADMB-FUBINACA) is a designer drug identified in synthetic cannabis blends in Japan in 2013. In 2018, it was the third-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Administration.

SDB-006 is a drug that acts as a potent agonist for the cannabinoid receptors, with an EC50 of 19 nM for human CB2 receptors, and 134 nM for human CB1 receptors. It was discovered during research into the related compound SDB-001 which had been sold illicitly as "2NE1". SDB-006 metabolism has been described in literature.

SDB-005 is an indazole-based synthetic cannabinoid that has been sold online as a designer drug. It is presumed to be an agonist of the CB1 and CB2 cannabinoid receptors. SDB-005 is the indazole core analog of PB-22 where the 8-hydroxyquinoline has also been replaced with a naphthalene group.
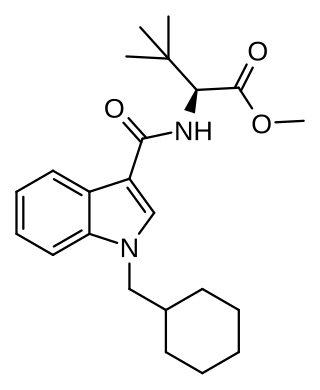
'MDMB-CHMICAa' is an indole-based synthetic cannabinoid that is a potent agonist of the CB1 receptor and has been sold online as a designer drug. While MDMB-CHMICA was initially sold under the name "MMB-CHMINACA", the compound corresponding to this code name (i.e. the isopropyl instead of t-butyl analogue of MDMB-CHMINACA) has been identified on the designer drug market in 2015 as AMB-CHMINACA.
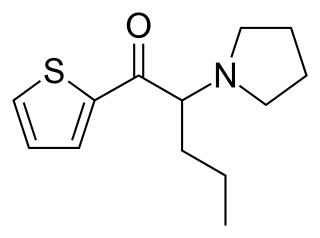
α-Pyrrolidinopentiothiophenone is a synthetic stimulant of the cathinone class that has been sold online as a designer drug. It is an analogue of α-PVP where the phenyl ring has been replaced by thiophene.
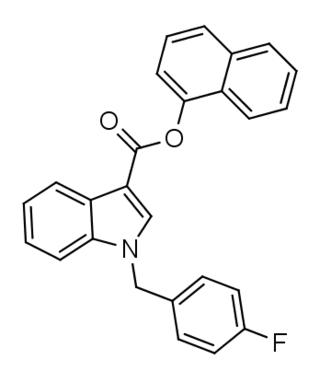
FDU-PB-22 is a derivative of JWH-018 that is presumed to be a potent agonist of the CB1 receptor, and has been sold online as a designer drug.
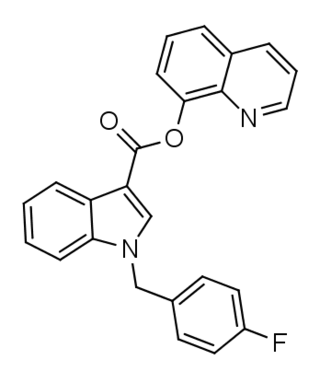
FUB-PB-22 (QUFUBIC) is an indole-based synthetic cannabinoid that is a potent agonist of the CB1 receptor and has been sold online as a designer drug.

QMPSB is an arylsulfonamide-based synthetic cannabinoid that has been sold as a designer drug.
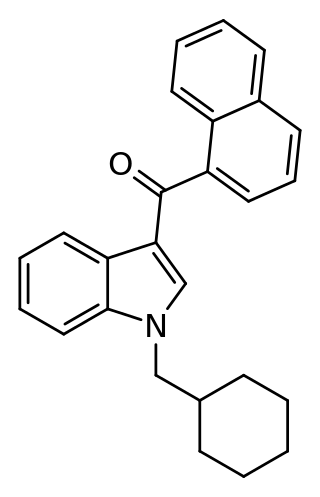
NE-CHMIMO (CHM-018) is an indole-based synthetic cannabinoid that is presumed to be a potent agonist of the CB1 receptor and has been sold online as a designer drug. NE-CHMIMO is the 1-cyclohexylmethyl (instead of 1-pentyl) analogue of the first-generation synthetic cannabinoid JWH-018. The corresponding cyclohexylmethyl derivative of JWH-081 had also been reported several months earlier.



















