
Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 100 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Cannabidiol (CBD) is a phytocannabinoid, one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. Medically, it is an anticonvulsant used to treat multiple forms of epilepsy. It was discovered in 1940 and, as of 2024 clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient high-quality evidence that CBD is effective for these conditions. CBD is sold as an herbal dietary supplement and promoted with yet unproven claims of particular therapeutic effects.

Tetrahydrocannabivarin is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of pentyl (5-carbon), making it non-psychoactive in lower doses. It has been shown to exhibit neuroprotective activity, appetite suppression, glycemic control and reduced side effects compared to THC, making it a potential treatment for management of obesity and diabetes. THCV was studied by Roger Adams as early as 1942.

Lenabasum (also known as ajulemic acid, 1',1'-dimethylheptyl-delta-8-tetrahydrocannabinol-11-oic acid, DMH-D8-THC-11-OIC, AB-III-56, HU-239, IP-751, CPL 7075, CT-3, JBT-101, Anabasum, and Resunab) is a synthetic cannabinoid that shows anti-fibrotic and anti-inflammatory effects in pre-clinical studies without causing a subjective "high". Although its design was inspired by a metabolite of delta-9-THC known as delta-9-THC-11-oic acid, lenabasum is an analog of the delta-8-THC metabolite delta-8-THC-11-oic acid. It is being developed for the treatment of inflammatory and fibrotic conditions such as systemic sclerosis, dermatomyositis and cystic fibrosis. It does not share the anti-emetic effects of some other cannabinoids, but may be useful for treating chronic inflammatory conditions where inflammation fails to resolve. Side effects include dry mouth, tiredness, and dizziness. The mechanism of action is through activation of the CB2 receptor leading to production of specialized proresolving eicosanoids such as lipoxin A4 and prostaglandin J2. Studies in animals at doses up to 40 mg/kg show minimal psychoactivity of lenabasum, compared to that produced by tetrahydrocannabinol. Lenabasum is being developed by Corbus Pharmaceuticals (formerly JB Therapeutics) for the treatment of orphan chronic life-threatening inflammatory diseases. Development since been discontinued.

Cannabigerol (CBG) is a non-psychoactive cannabinoid and minor constituent of cannabis. It is one of more than 120 identified cannabinoids found in the plant genus Cannabis. The compound is the decarboxylated form of cannabigerolic acid (CBGA), the parent molecule from which other cannabinoids are biosynthesized.

N-Arachidonyl glycine receptor, also known as G protein-coupled receptor 18 (GPR18), is a protein that in humans is encoded by the GPR18 gene. Along with the other previously orphan receptors GPR55 and GPR119, GPR18 has been found to be a receptor for endogenous lipid neurotransmitters, several of which also bind to cannabinoid receptors. It has been found to be involved in the regulation of intraocular pressure.

G protein-coupled receptor 55 also known as GPR55 is a G protein-coupled receptor that in humans is encoded by the GPR55 gene.
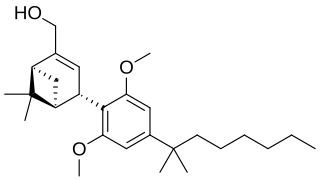
Onternabez (also known as HU-308, HU308, PPP-003, and ARDS-003) is a synthetic cannabinoid that acts as a potent cannabinoid agonist. It is highly selective for the cannabinoid-2 receptor (CB2 receptor) subtype, with a selectivity more than 5,000 times greater for the CB2 receptor than the CB1 receptor. The synthesis and characterization of onternabez took place in the laboratory of Raphael Mechoulam at the Hebrew University of Jerusalem (the HU in HU-308) in the late 1990s. The pinene dimethoxy-DMH-CBD derivative onternabez was identified as a potent peripheral CB2-selective agonist in in vitro and animal studies in 1990 and 1999.

Cannabichromene (CBC), also called cannabichrome, cannanbichromene, pentylcannabichromene or cannabinochromene, exhibits anti-inflammatory properties in vitro, which may, theoretically, contribute to cannabis analgesic effects. It is a phytocannabinoid, one of the hundreds of cannabinoids found in the Cannabis plant. It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol (THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabinol (CBN), among others. CBC and cannabinols are present in cannabis. It is not scheduled by the Convention on Psychotropic Substances.

Abnormal cannabidiol (Abn-CBD) is a synthetic regioisomer of cannabidiol, which unlike most other cannabinoids produces vasodilator effects, lowers blood pressure, and induces cell migration, cell proliferation and mitogen-activated protein kinase activation in microglia, but without producing any psychoactive or sedative effects. Abn-CBD can be found as an impurity in synthetic cannabidiol.

O-1602 is a synthetic compound most closely related to abnormal cannabidiol, and more distantly related in structure to cannabinoid drugs such as THC. O-1602 does not bind to the classical cannabinoid receptors CB1 or CB2 with any significant affinity, but instead is an agonist at several other receptors which appear to be related to the cannabinoid receptors, particularly GPR18 and GPR55. These previously orphan receptors have been found to be targets for a number of endogenous and synthetic cannabinoid compounds, and are thought to be responsible for most of the non-CB1, non-CB2 mediated effects that have become evident in the course of cannabinoid research. O-1602 produces some effects shared with classical cannabinoid compounds such as analgesic and antiinflammatory effects and appetite stimulation, but it does not produce sedation or psychoactive effects, and has several actions in the gut and brain that are not shared with typical cannabinoid agonists.
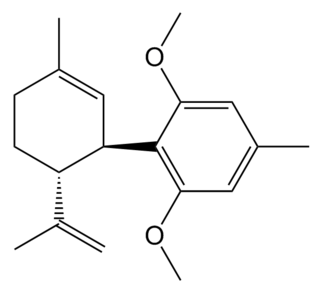
O-1918 is a synthetic compound related to cannabidiol, which is an antagonist at two former orphan receptors GPR18 and GPR55, that appear to be related to the cannabinoid receptors. O-1918 is used in the study of these receptors, which have been found to be targets for a number of endogenous and synthetic cannabinoid compounds, and are thought to be responsible for most of the non-CB1, non-CB2 mediated effects that have become evident in the course of cannabinoid research.
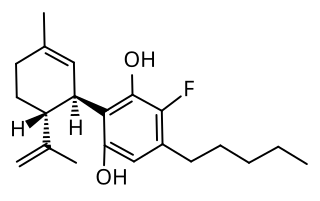
4'-Fluorocannabidiol is a fluorinated cannabidiol derivative that has more potent anxiolytic, antidepressant, antipsychotic and anti-compulsive activity in mice compared to its parent compound. It was first synthesized in 2016, alongside 10-fluorocannabidiol diacetate and 8,9-dihydro-7-fluorocannabidiol, which showed much weaker activity.

Cannabidiol-dimethylheptyl (CBD-DMH or DMH-CBD) is a synthetic homologue of cannabidiol where the pentyl chain has been replaced by a dimethylheptyl chain. Several isomers of this compound are known. The most commonly used isomer in research is (−)-CBD-DMH, which has the same stereochemistry as natural cannabidiol, and a 1,1-dimethylheptyl side chain. This compound is not psychoactive and acts primarily as an anandamide reuptake inhibitor, but is more potent than cannabidiol as an anticonvulsant and has around the same potency as an antiinflammatory. Unexpectedly the “unnatural” enantiomer (+)-CBD-DMH, which has reversed stereochemistry from cannabidiol, was found to be a directly acting cannabinoid receptor agonist with a Ki of 17.4nM at CB1 and 211nM at CB2, and produces typical cannabinoid effects in animal studies, as does its 7-OH derivative.
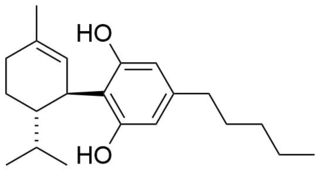
8,9-Dihydrocannabidiol is a synthetic cannabinoid that is closely related to cannabidiol (CBD) itself. that was first synthesized by Alexander R. Todd in 1940 derived from the catalytic hydrogenation of cannabidiol.
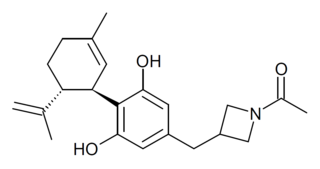
KLS-13019 is a cannabidiol derivative that has been modified on the side chain to improve solubility and tissue penetration properties. It was developed and patented by Neuropathix subsidiary Kannalife and found to be 50x more potent than cannabidiol as a neuroprotective agent, thought to be mediated by modulation of the sodium-calcium exchanger channel. It also had a higher therapeutic index than cannabidiol. Both KLS-13019 and cannabidiol, prevented the development of CIPN, while only KLS-13019 uniquely reversed neuropathic pain from chemotherapy. KLS-13019 binds to fewer biological targets than cannabidiol and KLS-13019 may possess the unique ability to reverse addictive behaviour, an effect not observed with cannabidiol.

7-Hydroxycannabidiol (7-OH-CBD) is an active metabolite of cannabidiol, generated in the body from cannabidiol by the action of the enzyme CYP2C19. While methods have been developed for its synthetic production, and measurement of levels in the body following consumption of cannabidiol, its pharmacology has been relatively little studied, though it has been found to possess similar anticonvulsant effects to cannabidiol itself, as well as lowering blood triglyceride levels. Like its precursor CBD, it is not known to exhibit any psychoactive effects on the body and is known to counter the psychoactive effects of THC if it is present at the same time. This mode of action in 2015 was discovered to be at least contributing in part by being a non competitive negative allosteric modulator of the Cannabinoid receptor type 1.

H4CBD is a cannabinoid that was first synthesized by Alexander R. Todd in 1940 derived from the catalytic hydrogenation of cannabidiol.

H2CBD are cannabinoids that were first synthesized by Alexander R. Todd in 1940 by catalytic hydrogenation of cannabidiol.

Etrinabdione (VCE-004.8, EHP-101) is a drug structurally related to cannabidiol and HU-331 which has potent antiinflammatory and neuroprotective effects. It acts as a dual agonist of CB2 and PPARγ and is in clinical trials for the treatment of scleroderma.




















