
Nicotine is a naturally produced alkaloid in the nightshade family of plants and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve withdrawal symptoms. Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits where it acts as a receptor antagonist.

Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Orexin, also known as hypocretin, is a neuropeptide that regulates arousal, wakefulness, and appetite. It exists in the forms of orexin-A and orexin-B. The most common form of narcolepsy, type 1, in which the individual experiences brief losses of muscle tone, is caused by a lack of orexin in the brain due to destruction of the cells that produce it.

Bupropion, formerly called amfebutamone, and sold under the brand name Wellbutrin among others, is an atypical antidepressant primarily used to treat major depressive disorder and to support smoking cessation. It is also popular as an add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant. Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction, it is not associated with weight gain and sleepiness, and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue. Bupropion, particularly the immediate release formulation, carries a higher risk of seizure than many other antidepressants, hence caution is recommended in patients with a history of seizure disorder. The medication is taken by mouth.
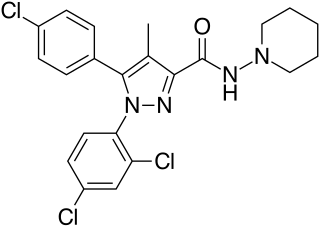
Rimonabant (also known as SR141716; trade names Acomplia, Zimulti) is an anorectic antiobesity drug approved in Europe in 2006 but was withdrawn worldwide in 2008 due to serious psychiatric side effects; it was never approved in the United States. Rimonabant is an inverse agonist for the cannabinoid receptor CB1 and was first-in-class for clinical development.

Tetrahydrocannabivarin is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of pentyl (5-carbon), making it non-psychoactive in lower doses. It has been shown to exhibit neuroprotective activity, appetite suppression, glycemic control and reduced side effects compared to THC, making it a potential treatment for management of obesity and diabetes. THCV was studied by Roger Adams as early as 1942.

Apomorphine, sold under the brand name Apokyn among others, is a type of aporphine having activity as a non-selective dopamine agonist which activates both D2-like and, to a much lesser extent, D1-like receptors. It also acts as an antagonist of 5-HT2 and α-adrenergic receptors with high affinity. The compound is historically a morphine decomposition product made by boiling morphine with concentrated acid, hence the -morphine suffix. Contrary to its name, apomorphine does not actually contain morphine or its skeleton, nor does it bind to opioid receptors. The apo- prefix relates to it being a morphine derivative ("[comes] from morphine").

The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are endogenous lipid-based retrograde neurotransmitters that bind to cannabinoid receptors, and cannabinoid receptor proteins that are expressed throughout the vertebrate central nervous system and peripheral nervous system. The endocannabinoid system remains under preliminary research, but may be involved in regulating physiological and cognitive processes, including fertility, pregnancy, pre- and postnatal development, various activity of immune system, appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.

Lobeline is a piperidine alkaloid found in a variety of plants, particularly those in the genus Lobelia, including Indian tobacco, Devil's tobacco, great lobelia, Lobelia chinensis, and Hippobroma longiflora. In its pure form, it is a white amorphous powder which is freely soluble in water.
A heteromer is something that consists of different parts; the antonym of homomeric. Examples are:

SB-277,011A is a drug which acts as a potent and selective dopamine D3 receptor antagonist, which is around 80-100x selective for D3 over D2, and lacks any partial agonist activity.

Cannabinoid receptor 1 (CB1), is a G protein-coupled cannabinoid receptor that in humans is encoded by the CNR1 gene. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by endogenous cannabinoids called endocannabinoids, a group of retrograde neurotransmitters that include lipids, such as anandamide and 2-arachidonoylglycerol (2-AG); plant phytocannabinoids, such as docosatetraenoylethanolamide found in wild daga, the compound THC which is an active constituent of the psychoactive drug cannabis; and synthetic analogs of THC. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin (THCV).

Neramexane is a drug related to memantine, which acts as an NMDA antagonist and has neuroprotective effects. It is being developed for various possible applications, including treatment of tinnitus, Alzheimer's disease, drug addiction and as an analgesic. Animal studies have also suggested antidepressant and nootropic actions so that this drug may be used for a wide range of potential applications. It also acts as a nicotinic acetylcholine receptor antagonist.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.
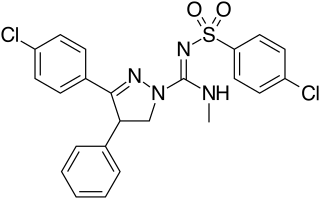
Ibipinabant (SLV319, BMS-646,256) is a drug used in scientific research which acts as a potent and highly selective CB1 antagonist. It has potent anorectic effects in animals, and was researched for the treatment of obesity, although CB1 antagonists as a class have now fallen out of favour as potential anorectics following the problems seen with rimonabant, and so ibipinabant is now only used for laboratory research, especially structure-activity relationship studies into novel CB1 antagonists. SLV330, which is a structural analogue of Ibipinabant, was reported active in animal models related to the regulation of memory, cognition, as well as in addictive behavior. An atom-efficient synthesis of ibipinabant has been reported.

SR144528 is a drug that acts as a potent and highly selective CB2 receptor inverse agonist, with a Ki of 0.6 nM at CB2 and 400 nM at the related CB1 receptor. It is used in scientific research for investigating the function of the CB2 receptor, as well as for studying the effects of CB1 receptors in isolation, as few CB1 agonists that do not also show significant activity as CB2 agonists are available. It has also been found to be an inhibitor of sterol O-acyltransferase, an effect that appears to be independent from its action on CB2 receptors.

AM-6545 is a drug which acts as a peripherally selective silent antagonist for the CB1 receptor, and was developed for the treatment of obesity. Other cannabinoid antagonists such as rimonabant have been marketed for this application, but have subsequently been withdrawn from sale because of centrally mediated side effects such as depression and nausea. Because AM-6545 does not cross the blood–brain barrier to any significant extent, it does not produce these kinds of side effects, but has still been shown to effectively reduce appetite and food consumption in animal studies.
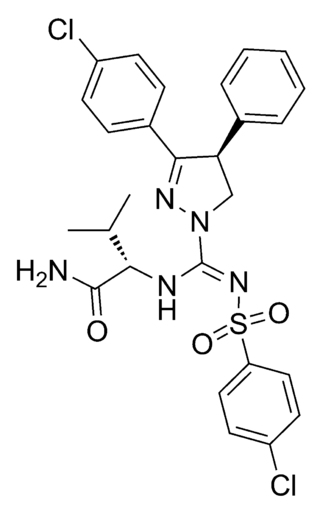
JD5037 is an antiobesity drug candidate which acts as a peripherally-restricted cannabinoid inverse agonist at CB1 receptors. It is very selective for the CB1 subtype, with a Ki of 0.35nM, >700-fold higher affinity than it has for CB2 receptors.

Aticaprant, also known by its developmental codes JNJ-67953964, CERC-501, and LY-2456302, is a κ-opioid receptor (KOR) antagonist which is under development for the treatment of major depressive disorder. A regulatory application for approval of the medication is expected to be submitted by 2025. Aticaprant is taken by mouth.
Jed Eugene Rose is an American academic professor, inventor and researcher in the field of nicotine and smoking cessation. Rose is presently the President and CEO of the Rose Research Center, LLC in Raleigh, North Carolina. Additionally, he is the Director of the Duke Center for Smoking Cessation at Duke University Medical Center.


















