This article may require cleanup to meet Wikipedia's quality standards. The specific problem is: significant portions of unreferenced, vague or incorrect information. Additionally, the areas that are cited are highly inappropriate citations.(August 2014) |
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 6α-Hydrocodol [1] |
| AHFS/Drugs.com | International Drug Names |
| Dependence liability | High |
| Addiction liability | High |
| Routes of administration |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | By mouth: 21% (range 12–34%) [3] |
| Metabolism | |
| Metabolites | • Dihydromorphine • Nordihydrocodeine • Others (e.g., conjugates) |
| Elimination half-life | 4 hours [3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.303 |
| Chemical and physical data | |
| Formula | C18H23NO3 |
| Molar mass | 301.386 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
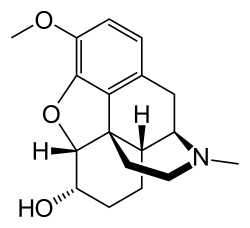
Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) (as in co-dydramol) or aspirin. It was developed in Germany in 1908 and first marketed in 1911. [4]
Contents
- Medical uses
- Side effects
- Pharmacology
- Regulation
- Chemistry
- Recreational use
- History
- Society and culture
- Brand names
- Preparations and availability
- References
- External links
Commonly available as tablets, solutions, elixirs, and other oral forms, dihydrocodeine is also available in some countries as an injectable solution for deep subcutaneous and intra-muscular administration. As with codeine, intravenous administration should be avoided, as it could result in anaphylaxis and life-threatening pulmonary edema. In the past, dihydrocodeine suppositories were used. Dihydrocodeine is available in suppository form on prescription. Dihydrocodeine is used as an alternative to codeine and similarly belongs to step 2 of the WHO analgesic ladder. [5]
It was first described in 1911 and approved for medical use in 1948. [6] Dihydrocodeine was developed during the search for more effective cough medication, especially to help reduce the spread of tuberculosis, pertussis, and pneumonia in the years from c.a. 1895 to 1915. It is similar in chemical structure to codeine.
