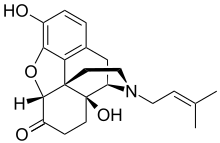
Morphine, formerly also called morphia, is a strong opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies. It is mainly used as an analgesic. There are numerous methods used to administer morphine: oral; sublingual; via inhalation; injection into a muscle, injection under the skin, or injection into the spinal cord area; transdermal; or via rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered by mouth, while the duration of its effect is 3–7 hours. Long-acting formulations of morphine are available as MS-Contin, Kadian, and other brand names as well as generically.
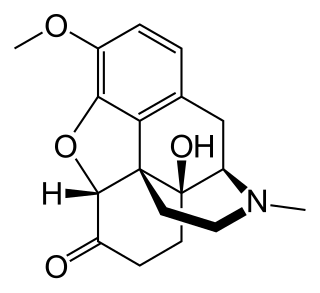
Oxycodone, sold under various brand names such as Roxicodone and OxyContin, is a semi-synthetic opioid used medically for treatment of moderate to severe pain. It is highly addictive and is a commonly abused drug. It is usually taken by mouth, and is available in immediate-release and controlled-release formulations. Onset of pain relief typically begins within fifteen minutes and lasts for up to six hours with the immediate-release formulation. In the United Kingdom, it is available by injection. Combination products are also available with paracetamol (acetaminophen), ibuprofen, naloxone, naltrexone, and aspirin.

Opioids are a class of drugs that derive from, or mimic, natural substances found in the opium poppy plant. Opioids work in the brain to produce a variety of effects, including pain relief. As a class of substances, they act on opioid receptors to produce morphine-like effects.

Buprenorphine, sold under the brand name Subutex among others, is an opioid used to treat opioid use disorder, acute pain, and chronic pain. It can be used under the tongue (sublingual), in the cheek (buccal), by injection, as a skin patch (transdermal), or as an implant. For opioid use disorder, the patient must have moderate opioid withdrawal symptoms before buprenorphine can be administered under direct observation of a health-care provider.

Oxymorphone is a highly potent opioid analgesic indicated for treatment of severe pain. Pain relief after injection begins after about 5–10 minutes, after oral administration it begins after about 30 minutes, and lasts about 3–4 hours for immediate-release tablets and 12 hours for extended-release tablets. The elimination half-life of oxymorphone is much faster intravenously, and as such, the drug is most commonly used orally. Like oxycodone, which metabolizes to oxymorphone, oxymorphone has a high potential to be abused.

Nalbuphine, sold under the brand names Nubain among others, is an opioid analgesic which is used in the treatment of pain. It is given by injection into a vein, muscle, or fat.
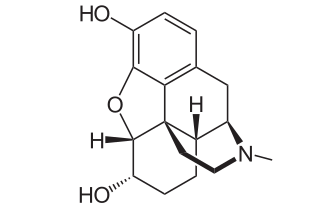
Dihydromorphine is a semi-synthetic opioid structurally related to and derived from morphine. The 7,8-double bond in morphine is reduced to a single bond to get dihydromorphine. Dihydromorphine is a moderately strong analgesic and is used clinically in the treatment of pain and also is an active metabolite of the analgesic opioid drug dihydrocodeine. Dihydromorphine occurs in trace quantities in assays of opium on occasion, as does dihydrocodeine, dihydrothebaine, tetrahydrothebaine, etc. The process for manufacturing dihydromorphine from morphine for pharmaceutical use was developed in Germany in the late 19th century, with the synthesis being published in 1900 and the drug introduced clinically as Paramorfan shortly thereafter. A high-yield synthesis from tetrahydrothebaine was later developed.

Nalorphine, also known as N-allylnormorphine, is a mixed opioid agonist–antagonist with opioid antagonist and analgesic properties. It was introduced in 1954 and was used as an antidote to reverse opioid overdose and in a challenge test to determine opioid dependence.

Oripavine is an opioid and the major metabolite of thebaine. It is the parent compound from which a series of semi-synthetic opioids are derived, which includes the compounds etorphine and buprenorphine. Although its analgesic potency is comparable to morphine, it is not used clinically due to its severe toxicity and low therapeutic index. Due to its use in manufacture of strong opioids, oripavine is a controlled substance in some jurisdictions.

Nalfurafine is an antipruritic that is marketed in Japan for the treatment of uremic pruritus in individuals with chronic kidney disease undergoing hemodialysis. It activates the κ-opioid receptor (KOR) and is potent, selective, and centrally active. It was the first selective KOR agonist approved for clinical use. It has also been dubiously referred to as the "first non-narcotic opioid drug" in history.
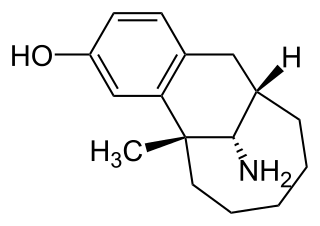
Dezocine, sold under the brand name Dalgan, is an atypical opioid analgesic which is used in the treatment of pain. It is used by intravenous infusion and intramuscular injection.
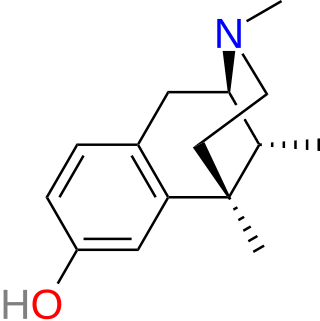
Metazocine is an opioid analgesic related to pentazocine. While metazocine has significant analgesic effects, mediated through a mixed agonist–antagonist action at the mu opioid receptor, its clinical use is limited by dysphoric and hallucinogenic effects which are most likely caused by activity at kappa opioid receptors and/or sigma receptors.

Propiram is a partial μ-opioid receptor agonist and weak μ antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

Oxilorphan is an opioid antagonist of the morphinan family that was never marketed. It acts as a μ-opioid receptor (MOR) antagonist but a κ-opioid receptor (KOR) partial agonist, and has similar effects to naloxone and around the same potency as an MOR antagonist. Oxilorphan has some weak partial agonist actions at the MOR and can produce hallucinogenic/dissociative effects at sufficient doses, indicative of KOR activation. It was trialed for the treatment of opioid addiction, but was not developed commercially. The KOR agonist effects of oxilorphan are associated with dysphoria, which combined with its hallucinogenic effects, serve to limit its clinical usefulness; indeed, many patients who experienced these side effects refused to take additional doses in clinical trials.
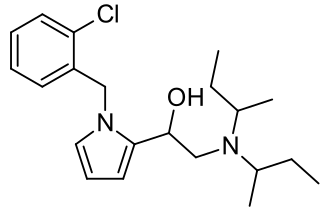
Viminol is an opioid analgesic developed by a team at the drug company Zambon in the 1960s. Viminol is based on the α-pyrryl-2-aminoethanol structure, unlike any other class of opioids.

RB-101 is a drug that acts as an enkephalinase inhibitor, which is used in scientific research.

Xorphanol (INN), also known as xorphanol mesylate (USAN), is an opioid analgesic of the morphinan family that was never marketed.

Oxymorphazone is an opioid analgesic drug related to oxymorphone. Oxymorphazone is a potent and long acting μ-opioid agonist which binds irreversibly to the receptor, forming a covalent bond which prevents it from detaching once bound. This gives it an unusual pharmacological profile, and while oxymorphazone is only around half the potency of oxymorphone, with higher doses the analgesic effect becomes extremely long lasting, with a duration of up to 48 hours. However, tolerance to analgesia develops rapidly with repeated doses, as chronically activated opioid receptors are rapidly internalised by β-arrestins, similar to the results of non-covalent binding by repeated doses of agonists with extremely high binding affinity such as lofentanil.

Alazocine, also known more commonly as N-allylnormetazocine (NANM), is a synthetic opioid analgesic of the benzomorphan family related to metazocine, which was never marketed. In addition to its opioid activity, the drug is a sigma receptor agonist, and has been used widely in scientific research in studies of this receptor. Alazocine is described as a potent analgesic, psychotomimetic or hallucinogen, and opioid antagonist. Moreover, one of its enantiomers was the first compound that was found to selectively label the σ1 receptor, and led to the discovery and characterization of the receptor.

An opiate is an alkaloid substance derived from opium. It differs from the similar term opioid in that the latter is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions, with evidence of opiate trade and use for pain relief as early as the eighth century AD. Most opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.