
Heroin, also known as diacetylmorphine and diamorphine among other names, is a morphinan opioid substance synthesized from the dried latex of the opium poppy; it is mainly used as a recreational drug for its euphoric effects. Heroin is used medically in several countries to relieve pain, such as during childbirth or a heart attack, as well as in opioid replacement therapy. Medical-grade diamorphine is used as a pure hydrochloride salt. Various white and brown powders sold illegally around the world as heroin are routinely diluted with cutting agents. Black tar heroin is a variable admixture of morphine derivatives—predominantly 6-MAM (6-monoacetylmorphine), which is the result of crude acetylation during clandestine production of street heroin.

Morphine, formerly also called morphia, is an opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies. It is mainly used as an analgesic. There are numerous methods used to administer morphine: oral; sublingual; via inhalation; injection into a muscle, injection under the skin, or injection into the spinal cord area; transdermal; or via rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered by mouth, while the duration of its effect is 3–7 hours. Long-acting formulations of morphine are sold under the brand names MS Contin and Kadian, among others. Generic long-acting formulations are also available.

Naloxone is an opioid antagonist: a medication used to reverse or reduce the effects of opioids. For example, it is used to restore breathing after an opioid overdose. Effects begin within two minutes when given intravenously, five minutes when injected into a muscle, and ten minutes as a nasal spray. Naloxone blocks the effects of opioids for 30 to 90 minutes.

Opioids are a class of drugs that derive from, or mimic, natural substances found in the opium poppy plant. Opioids work in the brain to produce a variety of effects, including pain relief. As a class of substances, they act on opioid receptors to produce morphine-like effects.

Buprenorphine, sold under the brand name Subutex among others, is an opioid used to treat opioid use disorder, acute pain, and chronic pain. It can be used under the tongue (sublingual), in the cheek (buccal), by injection, as a skin patch (transdermal), or as an implant. For opioid use disorder, the patient must have moderate opioid withdrawal symptoms before buprenorphine can be administered under direct observation of a health-care provider.

Naltrexone, sold under the brand name Revia among others, is a medication primarily used to manage alcohol use or opioid use disorder by reducing cravings and feelings of euphoria associated with substance use disorder. It has also been found effective in the treatment of other addictions and may be used for them off-label. An opioid-dependent person should not receive naltrexone before detoxification. It is taken orally or by injection into a muscle. Effects begin within 30 minutes, though a decreased desire for opioids may take a few weeks to occur.

Etorphine (M99) is a semi-synthetic opioid possessing an analgesic potency approximately 1,000–3,000 times that of morphine. It was first prepared in 1960 from oripavine, which does not generally occur in opium poppy extract but rather the related plants Papaver orientale and Papaver bracteatum. It was reproduced in 1963 by a research group at MacFarlan Smith in Gorgie, Edinburgh, led by Kenneth Bentley. It can be produced from thebaine.

Nalbuphine, sold under the brand names Nubain among others, is an opioid analgesic which is used in the treatment of pain. It is given by injection into a vein, muscle, or fat.

An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors.

Butorphanol is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers. Butorphanol is most closely structurally related to levorphanol. Butorphanol is available as the tartrate salt in injectable, tablet, and intranasal spray formulations. The tablet form is only used in dogs, cats and horses due to low bioavailability in humans.

Nicomorphine is the 3,6-dinicotinate ester of morphine. It is a strong opioid agonist analgesic two to three times as potent as morphine with a side effect profile similar to that of dihydromorphine, morphine, and diamorphine.

Dipropanoylmorphine is an opiate derivative, the 3,6-dipropanoyl ester of morphine. It was developed in 1972 as an analgesic. It is rarely used in some countries for the relief of severe pain such as that caused by terminal cancer, as an alternative to diamorphine (heroin) and morphine. The drug was first synthesised circa or about 1875 in Great Britain along with many other esters of morphine, all of which were shelved at the time, some of which were later developed such as heroin (1898), acetylpropionylmorphine (1924), dibenzoylmorphine, and so on. The name of this drug is also given as 3,6-dipropanoylmorphine and its 6-mono-acetylated homologue is also a longer-acting heroin-like drug, as are 3,6-diformylmorphine and 6-formylmorphine.
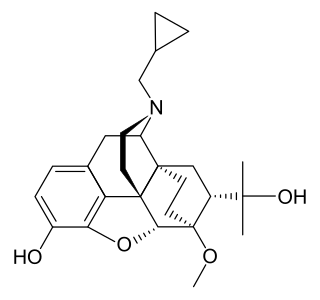
Diprenorphine, also known as diprenorfin, is a non-selective, high-affinity, weak partial agonist of the μ- (MOR), κ- (KOR), and δ-opioid receptor (DOR) which is used in veterinary medicine as an opioid antagonist. It is used to reverse the effects of super-potent opioid analgesics such as etorphine and carfentanil that are used for tranquilizing large animals. The drug is not approved for use in humans.

Nalorphine, also known as N-allylnormorphine, is a mixed opioid agonist–antagonist with opioid antagonist and analgesic properties. It was introduced in 1954 and was used as an antidote to reverse opioid overdose and in a challenge test to determine opioid dependence.

Oxilorphan is an opioid antagonist of the morphinan family that was never marketed. It acts as a μ-opioid receptor (MOR) antagonist but a κ-opioid receptor (KOR) partial agonist, and has similar effects to naloxone and around the same potency as an MOR antagonist. Oxilorphan has some weak partial agonist actions at the MOR and can produce hallucinogenic/dissociative effects at sufficient doses, indicative of KOR activation. It was trialed for the treatment of opioid addiction, but was not developed commercially. The KOR agonist effects of oxilorphan are associated with dysphoria, which combined with its hallucinogenic effects, serve to limit its clinical usefulness; indeed, many patients who experienced these side effects refused to take additional doses in clinical trials.

Levallorphan, also known as levallorphan tartrate (USAN), is an opioid modulator of the morphinan family used as an opioid analgesic and opioid antagonist/antidote. It acts as an antagonist of the μ-opioid receptor (MOR) and as an agonist of the κ-opioid receptor (KOR), and as a result, blocks the effects of stronger agents with greater intrinsic activity such as morphine whilst simultaneously producing analgesia.

Alazocine, also known more commonly as N-allylnormetazocine (NANM), is a synthetic opioid analgesic of the benzomorphan family related to metazocine, which was never marketed. In addition to its opioid activity, the drug is a sigma receptor agonist, and has been used widely in scientific research in studies of this receptor. Alazocine is described as a potent analgesic, psychotomimetic or hallucinogen, and opioid antagonist. Moreover, one of its enantiomers was the first compound that was found to selectively label the σ1 receptor, and led to the discovery and characterization of the receptor.
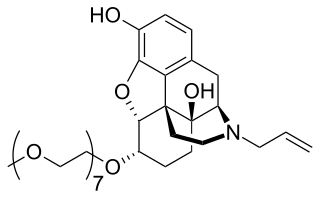
Naloxegol is a peripherally acting μ-opioid receptor antagonist developed by AstraZeneca, licensed from Nektar Therapeutics, for the treatment of opioid-induced constipation. It was approved in 2014 in adult patients with chronic, non-cancer pain. Doses of 25 mg were found safe and well tolerated for 52 weeks. When given concomitantly with opioid analgesics, naloxegol reduced constipation-related side effects, while maintaining comparable levels of analgesia.

Diacetylnalorphine (BAN), also known as O3,O6-diacetyl-N-allyl-normorphine, is an opioid drug described as an analgesic and antidote which was never marketed. It is the 3,6-diacetyl ester of nalorphine, and therefore the heroin analogue of nalorphine. Diacetylnalorphine may behave as a prodrug to nalorphine, similarly to the cases of heroin (diacetylmorphine) to morphine and diacetyldihydromorphine to dihydromorphine.

Nalodeine, also known more commonly as N-allylnorcodeine, is an opioid antagonist that was never marketed but is notable as the first opioid antagonist to be discovered. It was first reported in 1915 and was found to block the effects of morphine in animals. This was followed by the clinical introduction of nalorphine (N-allylnormorphine) in 1954, naloxone (N-allyloxymorphone) in 1960, and naltrexone (N-methylcyclopropyloxymorphone) in 1963. Nalmefene (6-desoxy-6-methylene-naltrexone), another structurally related opioid antagonist derivative, was also subsequently introduced, in 1996. In animals, nalodeine both reverses morphine- and heroin-induced respiratory depression and acts as a respiratory stimulant in its own right. Similarly to nalorphine, nalodeine has also been found to act as an agonist of the κ-opioid receptor.



















