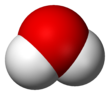
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen ion, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.

Sulfuric acid or sulphuric acid, known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is miscible with water.
In chemistry, hydronium is the cation [H3O]+, also written as H3O+, the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton to the surrounding water molecules. In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H+ and conjugate base.
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white solid that is practically insoluble in water. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities.
In chemistry, a superacid (according to the original definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (H2SO4), which has a Hammett acidity function (H0) of −12. According to the modern definition, a superacid is a medium in which the chemical potential of the proton is higher than in pure sulfuric acid. Commercially available superacids include trifluoromethanesulfonic acid (CF3SO3H), also known as triflic acid, and fluorosulfuric acid (HSO3F), both of which are about a thousand times stronger (i.e. have more negative H0 values) than sulfuric acid. Most strong superacids are prepared by the combination of a strong Lewis acid and a strong Brønsted acid. A strong superacid of this kind is fluoroantimonic acid. Another group of superacids, the carborane acid group, contains some of the strongest known acids. Finally, when treated with anhydrous acid, zeolites (microporous aluminosilicate minerals) will contain superacidic sites within their pores. These materials are used on massive scale by the petrochemical industry in the upgrading of hydrocarbons to make fuels.
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.

In chemistry, hydrogen halides are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, astatine, or tennessine. All known hydrogen halides are gases at standard temperature and pressure.
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide salt and occurs naturally as the rare mineral carobbiite. Solutions of KF will etch glass due to the formation of soluble fluorosilicates, although HF is more effective.

Fluorosulfuric acid is the inorganic compound with the chemical formula HSO3F. It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, H2SO4, substituting a fluorine atom for one of the hydroxyl groups. It is a colourless liquid, although commercial samples are often yellow.

Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides.

Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions. This mixture is a superacid that, in terms of corrosiveness, is trillions of times stronger than pure sulfuric acid when measured by its Hammett acidity function. It even protonates some hydrocarbons to afford pentacoordinate carbocations. Like its precursor hydrogen fluoride, it attacks glass, but can be stored in containers lined with PTFE (Teflon) or PFA.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Ammonium bifluoride is an inorganic compound with the formula [NH4][HF2] or [NH4]F·HF. It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-etchant and an intermediate in a once-contemplated route to hydrofluoric acid.
The bifluoride ion is an inorganic anion with the chemical formula [HF2]−. The anion is colorless. Salts of bifluoride are commonly encountered in the reactions of fluoride salts with hydrofluoric acid. The commercial production of fluorine involves electrolysis of bifluoride salts.
The Hammett acidity function (H0) is a measure of acidity that is used for very concentrated solutions of strong acids, including superacids. It was proposed by the physical organic chemist Louis Plack Hammett and is the best-known acidity function used to extend the measure of Brønsted–Lowry acidity beyond the dilute aqueous solutions for which the pH scale is useful.
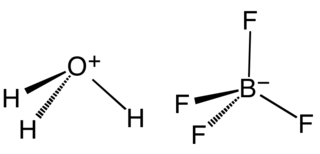
Fluoroboric acid or tetrafluoroboric acid is an inorganic compound with the simplified chemical formula H+[BF4]−. Solvent-free tetrafluoroboric acid has not been reported. The term "fluoroboric acid" usually refers to a range of compounds including hydronium tetrafluoroborate, which are available as solutions. The ethyl ether solvate is also commercially available, where the fluoroboric acid can be represented by the formula [H( 2O)n]+[BF4]−, where n is 2.
An acidity function is a measure of the acidity of a medium or solvent system, usually expressed in terms of its ability to donate protons to a solute. The pH scale is by far the most commonly used acidity function, and is ideal for dilute aqueous solutions. Other acidity functions have been proposed for different environments, most notably the Hammett acidity function, H0, for superacid media and its modified version H− for superbasic media. The term acidity function is also used for measurements made on basic systems, and the term basicity function is uncommon.
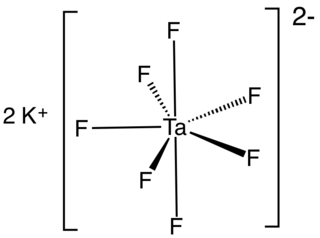
Potassium heptafluorotantalate is an inorganic compound with the formula K2[TaF7]. It is the potassium salt of the heptafluorotantalate anion [TaF7]2−. This white, water-soluble solid is an intermediate in the purification of tantalum from its ores and is the precursor to the metal.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.

Lutetium(III) fluoride is an inorganic compound with a chemical formula LuF3.