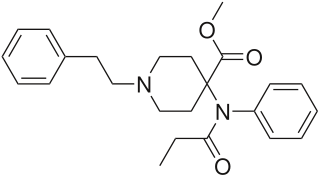
Carfentanil or carfentanyl, sold under the brand name Wildnil, is an extremely potent opioid analgesic used in veterinary medicine to anesthetize large animals such as elephants and rhinoceroses. It is typically administered in this context by tranquilizer dart. Carfentanil has also been used in humans to image opioid receptors. It has additionally been used as a recreational drug, typically by injection, insufflation, or inhalation. Deaths have been reported in association with carfentanil.

An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors.

The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP for its ligand ketazocine, is a G protein-coupled receptor that in humans is encoded by the OPRK1 gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction.

Nalfurafine is an antipruritic that is marketed in Japan for the treatment of uremic pruritus in individuals with chronic kidney disease undergoing hemodialysis. It activates the κ-opioid receptor (KOR) and is potent, selective, and centrally active. It was the first selective KOR agonist approved for clinical use. It has also been dubiously referred to as the "first non-narcotic opioid drug" in history.
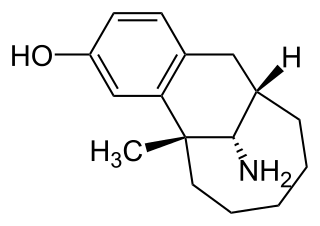
Dezocine, sold under the brand name Dalgan, is an atypical opioid analgesic which is used in the treatment of pain. It is used by intravenous infusion and intramuscular injection.

Tifluadom is a benzodiazepine derivative with an unusual activity profile. Unlike most benzodiazepines, tifluadom has no activity at the GABAA receptor, but instead is a selective agonist for the κ-opioid receptor. It has potent analgesic and diuretic effects in animals, and also has sedative effects and stimulates appetite.
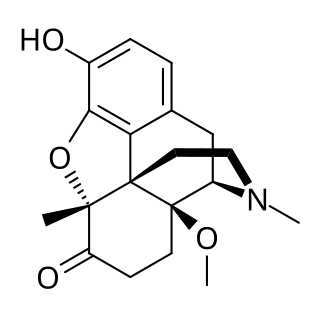
14-Methoxymetopon is an experimental opioid drug developed by a team led by Professor Helmut Schmidhammer at the University of Innsbruck in the mid-1990s. It is a derivative of metopon in which a methoxy group has been inserted at the 14-position. It is a highly potent analgesic drug that is around 500 times stronger than morphine when administered systemically; however, when given spinally or supraspinally, it exhibits analgesic activity up to a million fold greater than morphine. It binds strongly to the μ-opioid receptor and activates it to a greater extent than most similar opioid drugs. This produces an unusual pharmacological profile, and although 14-methoxymetopon acts as a potent μ-opioid full agonist in regard to some effects such as analgesia, a ceiling effect is seen on other effects such as constipation and respiratory depression which is believed to involve interaction with the κ-opioid receptor

Spiradoline (U-62066) is a drug which acts as a highly selective κ-opioid agonist. It has analgesic, diuretic, and antitussive effects, and produces subjective effects in animals similar to those of ketazocine and alazocine. The main effect in humans is sedation, along with analgesic and diuretic effects, but significant side effects such as dysphoria and hallucinations have stopped it from being used clinically.

HZ-2 is a drug which acts as a highly selective κ-opioid agonist. It is a potent analgesic with around the same potency as morphine, with a long duration of action and high oral bioavailability. Side effects include sedation, nausea and dysphoria as well as diuretic effects.
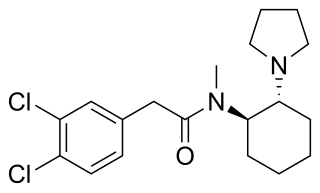
U-50488 is a drug which acts as a highly selective κ-opioid agonist, but without any μ-opioid antagonist effects. It has analgesic, diuretic and antitussive effects, and reverses the memory impairment produced by anticholinergic drugs. U-50488 was one of the first selective kappa agonists invented and research on its derivatives has led to the development of a large family of related compounds. This compound has never received FDA approval and there are no reported human cases in the literature involving an U-50488 overdose.
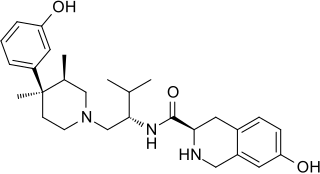
JDTic is a selective, long-acting ("inactivating") antagonist of the κ-opioid receptor (KOR). JDTic is a 4-phenylpiperidine derivative, distantly related structurally to analgesics such as pethidine and ketobemidone, and more closely to the MOR antagonist alvimopan. In addition, it is structurally distinct from other KOR antagonists such as norbinaltorphimine. JDTic has been used to create crystal structures of KOR [ PDB: 4DJH, 6VI4].

Alazocine, also known more commonly as N-allylnormetazocine (NANM), is a synthetic opioid analgesic of the benzomorphan family related to metazocine, which was never marketed. In addition to its opioid activity, the drug is a sigma receptor agonist, and has been used widely in scientific research in studies of this receptor. Alazocine is described as a potent analgesic, psychotomimetic or hallucinogen, and opioid antagonist. Moreover, one of its enantiomers was the first compound that was found to selectively label the σ1 receptor, and led to the discovery and characterization of the receptor.

GR-89696 is a drug which acts as a highly selective κ-opioid agonist. It has been studied in various animal species, and has been described as selective for the κ2 subtype. Recent studies have suggested that GR-89696 and related κ2-selective agonists may be useful for preventing the itching which is a common side effect of conventional opioid analgesic drugs, without the additional side effects of non-selective kappa agonists. The structure bound to the κ-opioid receptor has been reported.

BRL-52537 is a drug which acts as a potent and highly selective κ-opioid agonist. It has neuroprotective effects in animal studies, and is used for research into potential treatments for stroke and heart attack as well as more general brain research.

LPK-26 is a potent and selective κ-opioid agonist, and has analgesic effects.
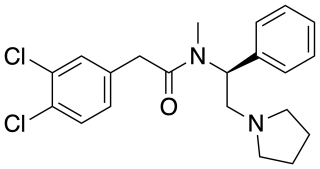
ICI-199,441 is a drug which acts as a potent and selective κ-opioid agonist, and has analgesic effects. It is a biased agonist of the KOR, and is one of relatively few KOR ligands that is G protein-biased rather than β-arrestin-biased.

U-47700, also known as U4, pink heroin, pinky, and pink, is an opioid analgesic drug developed by a team at Upjohn in the 1970s which has around 7.5 times the potency of morphine in animal models.
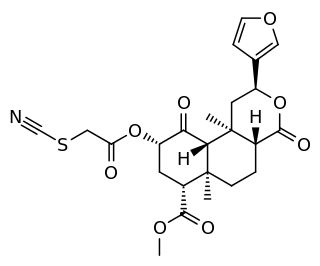
RB-64 is a semi-synthetic derivative of salvinorin A. It is an irreversible agonist, with a reactive thiocyanate group that forms a bond to the κ-opioid receptor (KOR), resulting in very high potency. It is functionally selective, activating G proteins more potently than β-arrestin-2. RB-64 has a bias factor of up to 96 and is analgesic with fewer of the side-effects associated with unbiased KOR agonists. The analgesia is long-lasting. Compared with unbiased agonists, RB-64 evokes considerably less receptor internalization.

Cebranopadol is an opioid analgesic of the benzenoid class which is currently under development internationally by Grünenthal, a German pharmaceutical company, and its partner Depomed, a pharmaceutical company in the United States, for the treatment of a variety of different acute and chronic pain states. As of November 2014, it is in phase III clinical trials.

HS665 is a drug which acts as a potent and selective κ-opioid receptor agonist, and has analgesic effects in animal studies. HS665 is not an agonist for the mu receptor, leading to less potential for abuse.





















