Related Research Articles

Cannabinoids are compounds found in cannabis. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is another major constituent of the plant. At least 113 distinct cannabinoids have been isolated from cannabis.
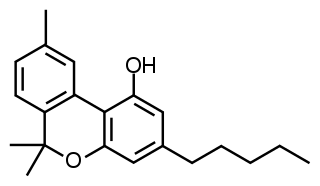
Cannabinol (CBN) is a mildly psychoactive cannabinoid found in trace amounts from Cannabis. CBN is mostly found in cannabis that is aged and stored, and is derived from the plant's main psychoactive chemical, tetrahydrocannabinol (THC).

HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended duration of action. HU-210 has a binding affinity of 0.061nM at CB1 and 0.52nM at CB2 in cloned human cannabinoid receptors. Compared to Delta-9-THC of 40.7nM at CB1. HU-210 is the (–)-1,1-dimethylheptyl analog of 11-hydroxy- Δ8- tetrahydrocannabinol; in some references it is called 1,1-dimethylheptyl- 11-hydroxytetrahydrocannabinol. The abbreviation "HU" stands for Hebrew University.

2-Arachidonoylglycerol (2-AG) is an endocannabinoid, an endogenous agonist of the CB1 receptor and the primary endogenous ligand for the CB2 receptor. It is an ester formed from the omega-6 fatty acid arachidonic acid and glycerol. It is present at relatively high levels in the central nervous system, with cannabinoid neuromodulatory effects. It has been found in maternal bovine and human milk. The chemical was first described in 1994-1995, although it had been discovered some time before that. The activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL) mediate its formation. 2-AG is synthesized from arachidonic acid-containing diacylglycerol (DAG).

JWH-073, a synthetic cannabinoid, is an analgesic chemical from the naphthoylindole family that acts as a partial agonist at both the CB1 and CB2 cannabinoid receptors. It is somewhat selective for the CB1 subtype, with affinity at this subtype approximately 5x the affinity at CB2. The abbreviation JWH stands for John W. Huffman, one of the inventors of the compound.

HU-308 (also known as onternabez, HU308, PPP-003 and ARDS-003) is a cannabidiol (CBD)-derivative drug that acts as a potent cannabinoid agonist. It is highly selective for the cannabinoid-2 receptor (CB2 receptor) subtype, with a selectivity more than 5,000 times greater for the CB2 receptor than the CB1 receptor. The synthesis and characterization of HU-308 took place in the laboratory of Raphael Mechoulam at the Hebrew University of Jerusalem (the HU in HU-308) in the late 1990s. The pinene dimethoxy-DMH-CBD derivative HU-308 was identified as a potent peripheral CB2-selective agonist in in vitro and animal studies in 1990 and 1999.

JWH-051 is an analgesic drug which is a cannabinoid agonist. Its chemical structure is closely related to that of the potent cannabinoid agonist HU-210, with the only difference being the removal of the hydroxyl group at position 1 of the aromatic ring. It was discovered and named after John W. Huffman.
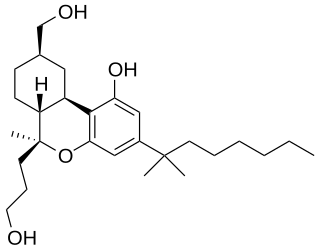
AM-919 is an analgesic drug which is a cannabinoid receptor agonist. It is a derivative of HU-210 which has been substituted with a 6β-(3-hydroxypropyl) group. This adds a "southern" aliphatic hydroxyl group to the molecule as seen in the CP-series of nonclassical cannabinoid drugs, and so AM-919 represents a hybrid structure between the classical dibenzopyran and nonclassical cannabinoid families.

Synthetic cannabinoids are a class of designer drug molecules that bind to the same receptors to which cannabinoids in cannabis plants attach. These novel psychoactive substances should not be confounded with synthetic phytocannabinoids or synthetic endocannabinoids from which they are in many aspects distinct.
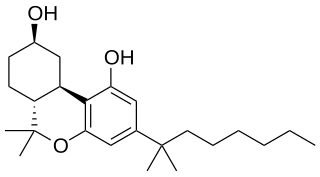
Canbisol (Nabidrox), is a synthetic cannabinoid derivative that is the dimethylheptyl homologue of 9-nor-9β-hydroxyhexahydrocannabinol (HHC). It is a potent agonist at both the CB1 and CB2 receptors, with a binding affinity of 0.1nM at CB1 and 0.2nM at CB2. It is mainly used in scientific research, in receptor binding studies to determine the structure and function of the cannabinoid receptors, but has been made illegal in some countries due to its possible abuse potential as a cannabinomimetic drug.

HU-243 (AM-4056) is a synthetic cannabinoid drug that is a single enantiomer of the hydrogenated derivative of the commonly used reference agonist HU-210. It is a methylene homologue of canbisol. It is a potent agonist at both the CB1 and CB2 receptors, with a binding affinity of 0.041 nM at the CB1 receptor, making it marginally more potent than HU-210, which had an affinity of 0.061 nM in the same assay.

AM-2233 is a drug that acts as a highly potent full agonist for the cannabinoid receptors, with a Ki of 1.8 nM at CB1 and 2.2 nM at CB2 as the active (R) enantiomer. It was developed as a selective radioligand for the cannabinoid receptors and has been used as its 131I derivative for mapping the distribution of the CB1 receptor in the brain. AM-2233 was found to fully substitute for THC in rats, with a potency lower than that of JWH-018 but higher than WIN 55,212-2.

HU-320 (7-nor-7-carboxy-CBD-1,1-DMH) is a drug related to cannabidiol, which has strong antiinflammatory and immunosuppressive properties while demonstrating no psychoactive effects.

HU-336 is a strongly antiangiogenic compound, significantly inhibiting angiogenesis at concentrations as low as 300 nM. It inhibits angiogenesis by directly inducing apoptosis of vascular endothelial cells without changing the expression of pro- and antiangiogenic cytokines and their receptors. HU-336 is highly effective against tumor xenografts in nude mice.

HU-345 is a drug that is able to inhibit aortic ring angiogenesis more potently than its parent compound cannabinol.

NESS-040C5 is a potent cannabinoid agonist which was developed for the treatment of glaucoma. It has reasonable selectivity for the CB2 receptor subtype, having a CB2 affinity of 0.4nM, and 25x selectivity over the related CB1 receptor.

Cannabidiol-dimethylheptyl (CBD-DMH or DMH-CBD) is a synthetic homologue of cannabidiol where the pentyl chain has been replaced by a dimethylheptyl chain. Several isomers of this compound are known. The most commonly used isomer in research is (−)-CBD-DMH, which has the same stereochemistry as natural cannabidiol, and a 1,1-dimethylheptyl side chain. This compound is not psychoactive and acts primarily as an anandamide reuptake inhibitor, but is more potent than cannabidiol as an anticonvulsant and has around the same potency as an antiinflammatory. Unexpectedly the “unnatural” enantiomer (+)-CBD-DMH, which has reversed stereochemistry from cannabidiol, was found to be a directly acting cannabinoid receptor agonist with a Ki of 17.4nM at CB1 and 211nM at CB2, and produces typical cannabinoid effects in animal studies, as does its 7-OH derivative.

(-)-Trans-Δ9-tetrahydrocannabiphorol (Δ9-THCP, (C7)-Δ9-THC, and THC-Heptyl), is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC, but for the first time in 2019 was isolated as a natural product in trace amounts from Cannabis sativa. It is structurally similar to Δ9-THC, the main active component of cannabis, but with the pentyl side chain extended to heptyl. Since it has a longer side chain, its cannabinoid effects are "far higher than Δ9-THC itself." It is said to have at least 30 times higher affinity to cannabinoid receptors than THC. The binding activity of Delta-9-THCP against human CB1 receptor in vitro is Ki = 1.2 nM. and the binding activity of Delta-8-THCP against human CB1 receptor in vitro is Ki = 22 nM.

11-Hydroxyhexahydrocannabinol is a minor active metabolite of tetrahydrocannabinol, and also a metabolite of the trace cannabinoid hexahydrocannabinol.
References
- 1 2 Howlett, AC; Champion, TM; Wilken, GH; Mechoulam, R (February 1990). "Stereochemical effects of 11-OH-delta 8-tetrahydrocannabinol-dimethylheptyl to inhibit adenylate cyclase and bind to the cannabinoid receptor". Neuropharmacology. 29 (2): 161–5. doi:10.1016/0028-3908(90)90056-W. PMID 2158635. S2CID 28602221.